当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective Diazine Dearomatization: The Catalytic Enantioselective Dearomatization of Pyrazine
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-15 , DOI: 10.1021/jacs.4c02979 Devin R. Ketelboeter 1 , Mukesh Pappoppula 1 , Aaron Aponick 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-15 , DOI: 10.1021/jacs.4c02979 Devin R. Ketelboeter 1 , Mukesh Pappoppula 1 , Aaron Aponick 1
Affiliation
|
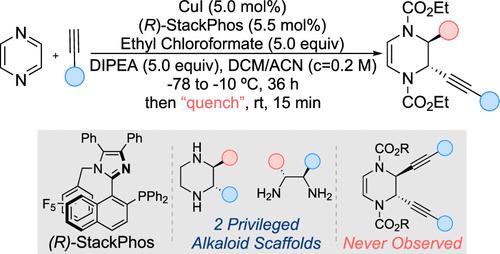
Despite much progress in the area of dearomatization, the enantioselective dearomatization of heterocycles is limited to those with a single heteroatom. Here we report a highly enantioselective copper-catalyzed dearomatization of pyrazine, a diazine, leading to chiral C-substituted piperazines. When exposed to a chloroformate and an alkyne in the presence of a catalyst derived from a copper salt and the chiral ligand StackPhos, pyrazine is readily dearomatized to provide a 2,3-disubstituted dihydropyrazine as single diastereomer in high enantiomeric excess. Mechanistic studies support a noninnocent involvement of chloride ion preventing a second iminium alkynylation, thus enabling subsequent functionalization at the second reactive site. The synthetically useful dihydropyrazine products, obtained in up to 95% yield and 99% ee, can be further manipulated to form optically active C-substituted piperazines and C1-symmetric 1,2-diamines.
中文翻译:

化学选择性二嗪脱芳构化:吡嗪的催化对映选择性脱芳构化
尽管在脱芳构化领域取得了很大进展,但杂环的对映选择性脱芳构化仅限于具有单个杂原子的杂环。在这里,我们报道了吡嗪(一种二嗪)的高度对映选择性铜催化脱芳构化,产生手性 C 取代哌嗪。当在铜盐和手性配体 StackPhos 衍生的催化剂存在下暴露于氯甲酸酯和炔烃时,吡嗪很容易脱芳构化,以高对映体过量提供单一非对映体形式的 2,3-二取代二氢吡嗪。机理研究支持氯离子的非无害参与阻止了第二次亚胺炔基化,从而实现了第二个反应位点的后续官能化。合成上有用的二氢吡嗪产物,以高达95%的收率和99% ee获得,可以进一步操作以形成光学活性的C-取代的哌嗪和C 1 -对称的1,2-二胺。
更新日期:2024-04-15
中文翻译:

化学选择性二嗪脱芳构化:吡嗪的催化对映选择性脱芳构化
尽管在脱芳构化领域取得了很大进展,但杂环的对映选择性脱芳构化仅限于具有单个杂原子的杂环。在这里,我们报道了吡嗪(一种二嗪)的高度对映选择性铜催化脱芳构化,产生手性 C 取代哌嗪。当在铜盐和手性配体 StackPhos 衍生的催化剂存在下暴露于氯甲酸酯和炔烃时,吡嗪很容易脱芳构化,以高对映体过量提供单一非对映体形式的 2,3-二取代二氢吡嗪。机理研究支持氯离子的非无害参与阻止了第二次亚胺炔基化,从而实现了第二个反应位点的后续官能化。合成上有用的二氢吡嗪产物,以高达95%的收率和99% ee获得,可以进一步操作以形成光学活性的C-取代的哌嗪和C 1 -对称的1,2-二胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号