当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Near infrared-emitting persistent luminescence nanoparticles@macrophages as cell-based carriers for precise imaging-guided cancer cell ablation
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2024-04-12 , DOI: 10.1039/d3nj02936k Hua Wang , Jie Zhang , Bin Zheng , Zirui Yang , Jiayi Sun , Xiao Liu , Niansong Qian
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2024-04-12 , DOI: 10.1039/d3nj02936k Hua Wang , Jie Zhang , Bin Zheng , Zirui Yang , Jiayi Sun , Xiao Liu , Niansong Qian
|
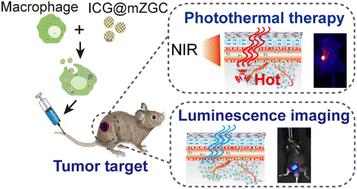
Conventional fluorescence-guided theranostics has a low signal-to-noise ratio and target efficiency that has limited its clinical application. To solve these problems, we developed near infrared-emitting persistent luminescence nanoparticles loaded mesoporous silica nanoparticles (mZGC) with macrophages acting as cellular carriers to mediate targeted delivery of these theranostic agents to the tumor area. The system can effectively improve the signal-to-noise ratio by reducing short-lived autofluorescent interference originating from in situ excitation, and the use of macrophages as cellular carriers for theranostic agent loading can significantly improve oncotherapeutic target efficiency. Furthermore, the photothermal properties of ICG@mZGC were evaluated, and an excellent photothermal performance was achieved, with a photothermal conversion efficiency of 23.29%. Therefore, the near infrared-emitting persistent luminescence nanoparticles@macrophage delivery system has potential applications as a phosphorescent drug carrier, and provides a novel paradigm for the ablation of various cancer cells.
中文翻译:

近红外发射持久发光纳米颗粒@巨噬细胞作为细胞载体用于精确成像引导癌细胞消融
传统的荧光引导治疗诊断学的信噪比和靶向效率较低,限制了其临床应用。为了解决这些问题,我们开发了近红外发射持久发光纳米颗粒,负载介孔二氧化硅纳米颗粒(mZGC),巨噬细胞作为细胞载体,介导这些治疗诊断剂靶向递送到肿瘤区域。该系统可以通过减少原位激发产生的短寿命自发荧光干扰来有效提高信噪比,并且使用巨噬细胞作为治疗诊断试剂负载的细胞载体可以显着提高肿瘤治疗靶标效率。此外,对ICG@mZGC的光热性能进行了评估,取得了优异的光热性能,光热转换效率为23.29%。因此,近红外发射持久发光纳米粒子@巨噬细胞递送策略具有作为磷光药物载体的潜在应用,并为各种癌细胞消融的治疗提供了新的范例。
更新日期:2024-04-12
中文翻译:

近红外发射持久发光纳米颗粒@巨噬细胞作为细胞载体用于精确成像引导癌细胞消融
传统的荧光引导治疗诊断学的信噪比和靶向效率较低,限制了其临床应用。为了解决这些问题,我们开发了近红外发射持久发光纳米颗粒,负载介孔二氧化硅纳米颗粒(mZGC),巨噬细胞作为细胞载体,介导这些治疗诊断剂靶向递送到肿瘤区域。该系统可以通过减少原位激发产生的短寿命自发荧光干扰来有效提高信噪比,并且使用巨噬细胞作为治疗诊断试剂负载的细胞载体可以显着提高肿瘤治疗靶标效率。此外,对ICG@mZGC的光热性能进行了评估,取得了优异的光热性能,光热转换效率为23.29%。因此,近红外发射持久发光纳米粒子@巨噬细胞递送策略具有作为磷光药物载体的潜在应用,并为各种癌细胞消融的治疗提供了新的范例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号