当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Detergents with Scalable Properties Identify Noncanonical Lipopolysaccharide Binding to Bacterial Inner Membrane Proteins
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-11 , DOI: 10.1021/jacs.3c14358 Leonhard H. Urner 1, 2 , Francesco Fiorentino 3 , Denis Shutin 2 , Joshua B. Sauer 2 , Mark T. Agasid 2 , Tarick J. El-Baba 2 , Jani R. Bolla 2, 4 , Phillip J. Stansfeld 5 , Carol V. Robinson 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-11 , DOI: 10.1021/jacs.3c14358 Leonhard H. Urner 1, 2 , Francesco Fiorentino 3 , Denis Shutin 2 , Joshua B. Sauer 2 , Mark T. Agasid 2 , Tarick J. El-Baba 2 , Jani R. Bolla 2, 4 , Phillip J. Stansfeld 5 , Carol V. Robinson 2
Affiliation
|
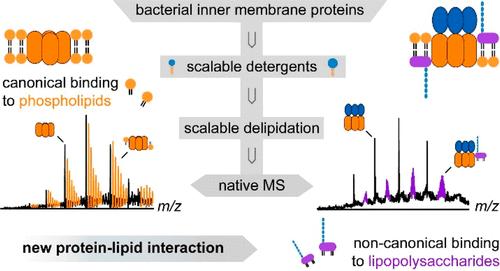
Lipopolysaccharide (LPS) is vital for maintaining the outer membrane barrier in Gram-negative bacteria. LPS is also frequently obtained in complex with the inner membrane proteins after detergent purification. The question of whether or not LPS binding to inner membrane proteins not involved in outer membrane biogenesis reflects native lipid environments remains unclear. Here, we leverage the control of the hydrophilic–lipophilic balance and packing parameter concepts to chemically tune detergents that can be used to qualitatively differentiate the degree to which proteins copurify with phospholipids (PLs) and/or LPS. Given the scalable properties of these detergents, we demonstrate a detergent fine-tuning that enables the facile investigation of intact proteins and their complexes with lipids by native mass spectrometry (nMS). We conclude that LPS, a lipid that is believed to be important for outer membranes, can also affect the activity of membrane proteins that are currently not assigned to be involved in outer membrane biogenesis. Our results deliver a scalable detergent chemistry for a streamlined biophysical characterization of protein–lipid interactions, provide a rationale for the high affinity of LPS-protein binding, and identify noncanonical associations between LPS and inner membrane proteins with relevance for membrane biology and antibiotic research.
中文翻译:

具有可扩展特性的洗涤剂可识别与细菌内膜蛋白结合的非典型脂多糖
脂多糖(LPS)对于维持革兰氏阴性菌的外膜屏障至关重要。 LPS 也经常在洗涤剂纯化后与内膜蛋白形成复合物。 LPS 与不参与外膜生物发生的内膜蛋白结合是否反映天然脂质环境的问题仍不清楚。在这里,我们利用亲水-亲脂平衡和堆积参数概念的控制来化学调节去垢剂,这些去垢剂可用于定性地区分蛋白质与磷脂(PL)和/或 LPS 共纯化的程度。鉴于这些去污剂的可扩展特性,我们展示了一种去污剂微调,可以通过天然质谱(nMS)轻松研究完整蛋白质及其与脂质的复合物。我们得出的结论是,LPS(一种被认为对外膜很重要的脂质)也可以影响目前未被指定参与外膜生物发生的膜蛋白的活性。我们的研究结果提供了一种可扩展的洗涤剂化学,用于简化蛋白质-脂质相互作用的生物物理表征,为LPS-蛋白质结合的高亲和力提供了原理,并确定了LPS和内膜蛋白质之间与膜生物学和抗生素研究相关的非典型关联。
更新日期:2024-04-11
中文翻译:

具有可扩展特性的洗涤剂可识别与细菌内膜蛋白结合的非典型脂多糖
脂多糖(LPS)对于维持革兰氏阴性菌的外膜屏障至关重要。 LPS 也经常在洗涤剂纯化后与内膜蛋白形成复合物。 LPS 与不参与外膜生物发生的内膜蛋白结合是否反映天然脂质环境的问题仍不清楚。在这里,我们利用亲水-亲脂平衡和堆积参数概念的控制来化学调节去垢剂,这些去垢剂可用于定性地区分蛋白质与磷脂(PL)和/或 LPS 共纯化的程度。鉴于这些去污剂的可扩展特性,我们展示了一种去污剂微调,可以通过天然质谱(nMS)轻松研究完整蛋白质及其与脂质的复合物。我们得出的结论是,LPS(一种被认为对外膜很重要的脂质)也可以影响目前未被指定参与外膜生物发生的膜蛋白的活性。我们的研究结果提供了一种可扩展的洗涤剂化学,用于简化蛋白质-脂质相互作用的生物物理表征,为LPS-蛋白质结合的高亲和力提供了原理,并确定了LPS和内膜蛋白质之间与膜生物学和抗生素研究相关的非典型关联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号