当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-based discovery of small molecule inhibitors of FKBP51-Hsp90 protein-protein interaction
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2024-03-28 , DOI: 10.1016/j.ejmech.2024.116356 Lisha Wang , Rajnish Kumar , Bengt Winblad , Pavel F. Pavlov
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2024-03-28 , DOI: 10.1016/j.ejmech.2024.116356 Lisha Wang , Rajnish Kumar , Bengt Winblad , Pavel F. Pavlov
|
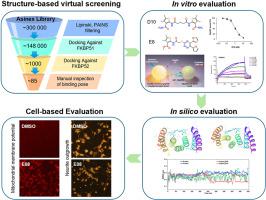
The heat shock protein 90 kDa (Hsp90) molecular chaperone machinery is responsible for the folding and activation of hundreds of important clients such as kinases, steroid hormone receptors, transcription factors, etc. This process is dynamically regulated in an ATP-dependent manner by Hsp90 co-chaperones including a group of tetratricopeptide (TPR) motif proteins that bind to the -terminus of Hsp90. Among these TPR containing co-chaperones, FK506-binding protein 51 kDa (FKBP51) is reported to play an important role in stress-related pathologies, psychiatric disorders, Alzheimer's disease, and cancer, making FKBP51-Hsp90 interaction a potential therapeutic target. In this study, we report identification of potent and selective inhibitors of FKBP51-Hsp90 protein-protein interaction using a structure-based virtual screening approach. Upon evaluation, the identified hits show a considerable degree of selectivity towards FKBP51 over other TPR proteins, particularly for highly homologous FKBP52. Tyr355 of FKBP51 emerged as an important contributor to inhibitor's specificity. Additionally, we demonstrate the impact of these inhibitors on cellular energy metabolism, and neurite outgrowth, which are subjects of FKBP51 regulation. Overall, the results from this study highlight a novel pharmacological approach towards regulation of FKBP51 function and more generally, Hsp90 function via its interaction with TPR co-chaperones.
中文翻译:

基于结构的 FKBP51-Hsp90 蛋白-蛋白相互作用小分子抑制剂的发现
热休克蛋白 90 kDa (Hsp90) 分子伴侣机制负责数百个重要客户的折叠和激活,如激酶、类固醇激素受体、转录因子等。该过程由 Hsp90 以 ATP 依赖性方式动态调节共伴侣包括一组与 Hsp90 的 β 末端结合的四肽 (TPR) 基序蛋白。据报道,在这些含有 TPR 的共伴侣中,FK506 结合蛋白 51 kDa (FKBP51) 在应激相关病理、精神疾病、阿尔茨海默病和癌症中发挥重要作用,使得 FKBP51-Hsp90 相互作用成为潜在的治疗靶点。在这项研究中,我们报告使用基于结构的虚拟筛选方法鉴定了 FKBP51-Hsp90 蛋白质-蛋白质相互作用的有效和选择性抑制剂。经过评估,与其他 TPR 蛋白相比,所识别的命中显示出对 FKBP51 的相当大程度的选择性,特别是对于高度同源的 FKBP52。 FKBP51 的 Tyr355 成为抑制剂特异性的重要贡献者。此外,我们还证明了这些抑制剂对细胞能量代谢和神经突生长的影响,这些都是 FKBP51 调节的对象。总体而言,这项研究的结果强调了一种新的药理学方法来调节 FKBP51 功能,更一般地说,通过与 TPR 共伴侣相互作用来调节 Hsp90 功能。
更新日期:2024-03-28
中文翻译:

基于结构的 FKBP51-Hsp90 蛋白-蛋白相互作用小分子抑制剂的发现
热休克蛋白 90 kDa (Hsp90) 分子伴侣机制负责数百个重要客户的折叠和激活,如激酶、类固醇激素受体、转录因子等。该过程由 Hsp90 以 ATP 依赖性方式动态调节共伴侣包括一组与 Hsp90 的 β 末端结合的四肽 (TPR) 基序蛋白。据报道,在这些含有 TPR 的共伴侣中,FK506 结合蛋白 51 kDa (FKBP51) 在应激相关病理、精神疾病、阿尔茨海默病和癌症中发挥重要作用,使得 FKBP51-Hsp90 相互作用成为潜在的治疗靶点。在这项研究中,我们报告使用基于结构的虚拟筛选方法鉴定了 FKBP51-Hsp90 蛋白质-蛋白质相互作用的有效和选择性抑制剂。经过评估,与其他 TPR 蛋白相比,所识别的命中显示出对 FKBP51 的相当大程度的选择性,特别是对于高度同源的 FKBP52。 FKBP51 的 Tyr355 成为抑制剂特异性的重要贡献者。此外,我们还证明了这些抑制剂对细胞能量代谢和神经突生长的影响,这些都是 FKBP51 调节的对象。总体而言,这项研究的结果强调了一种新的药理学方法来调节 FKBP51 功能,更一般地说,通过与 TPR 共伴侣相互作用来调节 Hsp90 功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号