当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accessing pyrrolo[1,2-a]indole derivatives via visible-light-induced dearomatizative cyclization of indoles
Chemical Communications ( IF 4.9 ) Pub Date : 2024-04-10 , DOI: 10.1039/d4cc01215a Zhaosheng Liu 1 , Xiaochen Ji 1 , Lilan Duan 1 , Guo-Jun Deng 1, 2 , Huawen Huang 1
Chemical Communications ( IF 4.9 ) Pub Date : 2024-04-10 , DOI: 10.1039/d4cc01215a Zhaosheng Liu 1 , Xiaochen Ji 1 , Lilan Duan 1 , Guo-Jun Deng 1, 2 , Huawen Huang 1
Affiliation
|
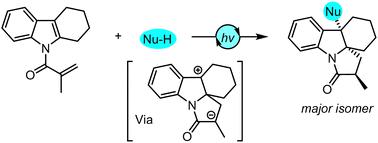
Pyrrolo[1,2-a]indoles are structurally important scaffolds in many natural products and bioactive compounds. Herein, we report a novel synthetic method for pyrrolo[1,2-a]indole derivatives through visible-light-induced cascade dearomatizative cyclization of indoles with external nucleophiles. Moderate yields, good diastereoselectivities, and excellent regioselectivities were generally observed with the resultant indole-fused polycyclic compounds.
中文翻译:

通过可见光诱导吲哚脱芳环化获得吡咯并[1,2-a]吲哚衍生物
吡咯并[1,2- a ]吲哚是许多天然产物和生物活性化合物中结构上重要的支架。在此,我们报告了一种通过可见光诱导吲哚与外部亲核试剂级联脱芳构环化合成吡咯并[1,2- a ]吲哚衍生物的新方法。所得吲哚稠合多环化合物通常观察到中等产率、良好的非对映选择性和优异的区域选择性。
更新日期:2024-04-15
中文翻译:

通过可见光诱导吲哚脱芳环化获得吡咯并[1,2-a]吲哚衍生物
吡咯并[1,2- a ]吲哚是许多天然产物和生物活性化合物中结构上重要的支架。在此,我们报告了一种通过可见光诱导吲哚与外部亲核试剂级联脱芳构环化合成吡咯并[1,2- a ]吲哚衍生物的新方法。所得吲哚稠合多环化合物通常观察到中等产率、良好的非对映选择性和优异的区域选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号