当前位置:
X-MOL 学术
›
Comput. Theor. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular dynamics simulations to evaluate the decomposition properties of methane hydrate under different thermodynamic conditions
Computational and Theoretical Chemistry ( IF 2.8 ) Pub Date : 2024-04-01 , DOI: 10.1016/j.comptc.2024.114585 Yanxiao Hei , Zilong Liu , Di Shi , Xin Wang , Xiaoliang Sun , Wenxiu Leng , Xue Li
Computational and Theoretical Chemistry ( IF 2.8 ) Pub Date : 2024-04-01 , DOI: 10.1016/j.comptc.2024.114585 Yanxiao Hei , Zilong Liu , Di Shi , Xin Wang , Xiaoliang Sun , Wenxiu Leng , Xue Li
|
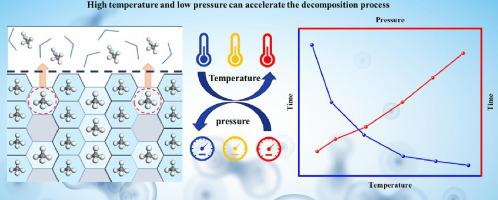
Understanding the thermodynamic effect on methane hydrate decomposition is beneficial for designing controllable methane recovery processes under conditions of continental margins and oceanic sediments. In this work, we systematically examined the effects of varying temperatures and pressures on the natural gas hydrate decomposition in the presence of 20 mol% methanol through the molecular dynamics approach. Taking advantages of key parameters of angular order parameter (AOP), radial distribution function (RDF), mean square displacement (MSD), and potential energy, we mainly aimed to explore the impact of temperature and pressure on the decomposition of methane hydrate. It can be founded that high temperature is a positive factor for the methane hydrate decomposition, and as the temperature increases from 272.15 K to 277.15 K, the promotion effect becomes more obvious, resulting in a reduction of decomposition time by 3.93 ns. On the contrary, pressure has a negative effect on the methane hydrate decomposition, and as the pressure increases from 1 bar to 200 bar, the inhibitory effect becomes smaller. Additionally, the potential energy as a function of time can be used to evaluate the decomposition rates under different thermodynamic conditions. The obtained results underscore the significant dependence of methane hydrate decomposition rates on temperature and pressure, providing essential guidelines for optimizing gas hydrate decomposition processes.
中文翻译:

分子动力学模拟评估不同热力学条件下甲烷水合物的分解特性
了解甲烷水合物分解的热力学效应有利于设计大陆边缘和海洋沉积物条件下的可控甲烷回收过程。在这项工作中,我们通过分子动力学方法系统地研究了在 20 mol% 甲醇存在下,不同温度和压力对天然气水合物分解的影响。利用角序参数(AOP)、径向分布函数(RDF)、均方位移(MSD)和势能等关键参数,我们主要探讨温度和压力对甲烷水合物分解的影响。可以发现,高温是甲烷水合物分解的积极因素,随着温度从272.15 K升高到277.15 K,促进作用更加明显,分解时间减少了3.93 ns。相反,压力对甲烷水合物分解有负面影响,随着压力从1 bar增加到200 bar,抑制作用变小。此外,势能作为时间的函数可用于评估不同热力学条件下的分解速率。获得的结果强调了甲烷水合物分解速率对温度和压力的显着依赖性,为优化天然气水合物分解过程提供了重要指导。
更新日期:2024-04-01
中文翻译:

分子动力学模拟评估不同热力学条件下甲烷水合物的分解特性
了解甲烷水合物分解的热力学效应有利于设计大陆边缘和海洋沉积物条件下的可控甲烷回收过程。在这项工作中,我们通过分子动力学方法系统地研究了在 20 mol% 甲醇存在下,不同温度和压力对天然气水合物分解的影响。利用角序参数(AOP)、径向分布函数(RDF)、均方位移(MSD)和势能等关键参数,我们主要探讨温度和压力对甲烷水合物分解的影响。可以发现,高温是甲烷水合物分解的积极因素,随着温度从272.15 K升高到277.15 K,促进作用更加明显,分解时间减少了3.93 ns。相反,压力对甲烷水合物分解有负面影响,随着压力从1 bar增加到200 bar,抑制作用变小。此外,势能作为时间的函数可用于评估不同热力学条件下的分解速率。获得的结果强调了甲烷水合物分解速率对温度和压力的显着依赖性,为优化天然气水合物分解过程提供了重要指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号