当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanodrug modified with engineered cell membrane targets CDKs to activate aPD-L1 immunotherapy against liver metastasis of immune-desert colon cancer
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2024-04-03 , DOI: 10.1016/j.jconrel.2024.03.052 Dongbing Ding , Rongpu Liang , Tan Li , Tianyun Lan , Yiquan Li , Shengxin Huang , Guanhui He , Jiannan Ren , Weibo Li , Zongheng Zheng , Tufeng Chen , Jiafeng Fang , Lijun Huang , Xintao Shuai , Bo Wei
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2024-04-03 , DOI: 10.1016/j.jconrel.2024.03.052 Dongbing Ding , Rongpu Liang , Tan Li , Tianyun Lan , Yiquan Li , Shengxin Huang , Guanhui He , Jiannan Ren , Weibo Li , Zongheng Zheng , Tufeng Chen , Jiafeng Fang , Lijun Huang , Xintao Shuai , Bo Wei
|
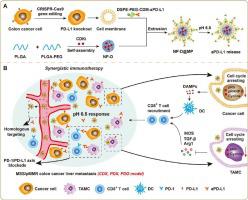
Immunotherapy based on the PD-1/PD-L1 axis blockade has no benefit for patients diagnosed with colon cancer liver metastasis (CCLM) for the microsatellite stable/proficient mismatch repair (MSS/pMMR)) subtype, which is known as an immune-desert cancer featuring poor immunogenicity and insufficient CD8 T cell infiltration in the tumor microenvironment. Here, a multifunctional nanodrug carrying a cyclin-dependent kinase (CDK)1/2/5/9 inhibitor and PD-L1 antibody is prepared to boost the immune checkpoint blockade (ICB)-based immunotherapy against MSS/pMMR CCLM reversing the immunosuppressive tumor microenvironment. To enhance the MSS/pMMR CCLM-targeting efficacy, we modify the nanodrug with PD-L1 knockout cell membrane of this colon cancer subtype. First, CDKs inhibitor delivered by nanodrug down-regulates phosphorylated retinoblastoma and phosphorylated RNA polymerase II and meanwhile arrests the G2/M cell cycle in CCLM to promote immunogenic signal release, stimulate dendritic cell maturation, and enhance CD8 T cell infiltration. Moreover, CDKi suppresses the secretion of immunosuppressive cytokines in tumor-associated myeloid cells sensitizing ICB therapy in CCLM. Notably, the great efficacy to activate immune responses is demonstrated in the patient-derived xenograft model and the patient-derived organoid model as well, revealing a clinical application potential. Overall, our study represents a promising therapeutic approach for targeting liver metastasis, remolding the tumor immune microenvironment (TIME), and enhancing the response of MSS/pMMR CCLM to boost ICB immunotherapy.
中文翻译:

用工程细胞膜修饰的纳米药物靶向 CDK,激活 aPD-L1 免疫疗法,对抗免疫沙漠结肠癌的肝转移
基于 PD-1/PD-L1 轴阻断的免疫治疗对于诊断为微卫星稳定/熟练错配修复 (MSS/pMMR) 亚型的结肠癌肝转移 (CCLM) 患者没有任何益处,该亚型被称为免疫-沙漠癌的特点是免疫原性差,肿瘤微环境中CD8 T细胞浸润不足。在这里,制备了一种携带细胞周期蛋白依赖性激酶(CDK)1/2/5/9抑制剂和PD-L1抗体的多功能纳米药物,以增强基于免疫检查点阻断(ICB)的针对MSS/pMMR CCLM的免疫疗法,从而逆转免疫抑制肿瘤微环境。为了增强 MSS/pMMR CCLM 靶向功效,我们用这种结肠癌亚型的 PD-L1 敲除细胞膜修饰纳米药物。首先,纳米药物递送的CDKs抑制剂下调磷酸化视网膜母细胞瘤和磷酸化RNA聚合酶II,同时阻滞CCLM中的G2/M细胞周期,促进免疫原性信号释放,刺激树突状细胞成熟,并增强CD8 T细胞浸润。此外,CDKi 抑制肿瘤相关骨髓细胞中免疫抑制细胞因子的分泌,使 CCLM 中的 ICB 治疗敏感。值得注意的是,在患者来源的异种移植模型和患者来源的类器官模型中也证明了激活免疫反应的巨大功效,揭示了临床应用潜力。总体而言,我们的研究代表了一种有前途的治疗方法,用于靶向肝转移、重塑肿瘤免疫微环境(TIME)以及增强 MSS/pMMR CCLM 的反应以增强 ICB 免疫治疗。
更新日期:2024-04-03
中文翻译:

用工程细胞膜修饰的纳米药物靶向 CDK,激活 aPD-L1 免疫疗法,对抗免疫沙漠结肠癌的肝转移
基于 PD-1/PD-L1 轴阻断的免疫治疗对于诊断为微卫星稳定/熟练错配修复 (MSS/pMMR) 亚型的结肠癌肝转移 (CCLM) 患者没有任何益处,该亚型被称为免疫-沙漠癌的特点是免疫原性差,肿瘤微环境中CD8 T细胞浸润不足。在这里,制备了一种携带细胞周期蛋白依赖性激酶(CDK)1/2/5/9抑制剂和PD-L1抗体的多功能纳米药物,以增强基于免疫检查点阻断(ICB)的针对MSS/pMMR CCLM的免疫疗法,从而逆转免疫抑制肿瘤微环境。为了增强 MSS/pMMR CCLM 靶向功效,我们用这种结肠癌亚型的 PD-L1 敲除细胞膜修饰纳米药物。首先,纳米药物递送的CDKs抑制剂下调磷酸化视网膜母细胞瘤和磷酸化RNA聚合酶II,同时阻滞CCLM中的G2/M细胞周期,促进免疫原性信号释放,刺激树突状细胞成熟,并增强CD8 T细胞浸润。此外,CDKi 抑制肿瘤相关骨髓细胞中免疫抑制细胞因子的分泌,使 CCLM 中的 ICB 治疗敏感。值得注意的是,在患者来源的异种移植模型和患者来源的类器官模型中也证明了激活免疫反应的巨大功效,揭示了临床应用潜力。总体而言,我们的研究代表了一种有前途的治疗方法,用于靶向肝转移、重塑肿瘤免疫微环境(TIME)以及增强 MSS/pMMR CCLM 的反应以增强 ICB 免疫治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号