当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Shortening CN⋯Br–Csp3 halogen bonds via π-stacking
CrystEngComm ( IF 3.1 ) Pub Date : 2024-04-01 , DOI: 10.1039/d4ce00307a Kamil Kupietz 1 , Rosa M. Gomila 2 , Thierry Roisnel 1 , Antonio Frontera 2 , Rafael Gramage-Doria 1
CrystEngComm ( IF 3.1 ) Pub Date : 2024-04-01 , DOI: 10.1039/d4ce00307a Kamil Kupietz 1 , Rosa M. Gomila 2 , Thierry Roisnel 1 , Antonio Frontera 2 , Rafael Gramage-Doria 1
Affiliation
|
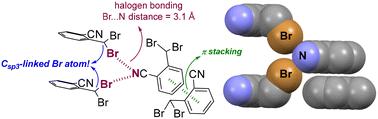
The X-ray structure of 2-(dibromomethyl)benzonitrile, featuring unexpectedly short C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯Br halogen bond distances between a nitrile group and a C(sp3)-linked bromine atom, is presented. Despite the weak Lewis base nature of the nitrile N atom and absence of strong electron-withdrawing groups on the Br atom, π-stacking significantly enhances both the electron donor and acceptor capability of the interacting partners. As such, and beyond trivial (hetero)aromatic systems, the rationale of CN⋯Br halogen bonding can also be employed in purely aliphatic systems.
N⋯Br halogen bond distances between a nitrile group and a C(sp3)-linked bromine atom, is presented. Despite the weak Lewis base nature of the nitrile N atom and absence of strong electron-withdrawing groups on the Br atom, π-stacking significantly enhances both the electron donor and acceptor capability of the interacting partners. As such, and beyond trivial (hetero)aromatic systems, the rationale of CN⋯Br halogen bonding can also be employed in purely aliphatic systems.
中文翻译:

通过π堆积缩短CN⋯Br–Csp3卤素键
提出了2-(二溴甲基)苯甲腈的 X 射线结构,其特征是腈基团和 C(sp 3![[三键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) ) 连接的溴原子之间具有出人意料的短 C N⋯Br 卤素键距离。尽管腈 N 原子的路易斯碱性质较弱且 Br 原子上不存在强吸电子基团,但 π 堆积显着增强了相互作用伙伴的电子供体和受体能力。因此,除了普通(杂)芳香族系统之外,C N⋯B r 卤素键合的基本原理也可以用于纯脂肪族系统。
) 连接的溴原子之间具有出人意料的短 C N⋯Br 卤素键距离。尽管腈 N 原子的路易斯碱性质较弱且 Br 原子上不存在强吸电子基团,但 π 堆积显着增强了相互作用伙伴的电子供体和受体能力。因此,除了普通(杂)芳香族系统之外,C N⋯B r 卤素键合的基本原理也可以用于纯脂肪族系统。
更新日期:2024-04-01
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯Br halogen bond distances between a nitrile group and a C(sp3)-linked bromine atom, is presented. Despite the weak Lewis base nature of the nitrile N atom and absence of strong electron-withdrawing groups on the Br atom, π-stacking significantly enhances both the electron donor and acceptor capability of the interacting partners. As such, and beyond trivial (hetero)aromatic systems, the rationale of CN⋯Br halogen bonding can also be employed in purely aliphatic systems.
N⋯Br halogen bond distances between a nitrile group and a C(sp3)-linked bromine atom, is presented. Despite the weak Lewis base nature of the nitrile N atom and absence of strong electron-withdrawing groups on the Br atom, π-stacking significantly enhances both the electron donor and acceptor capability of the interacting partners. As such, and beyond trivial (hetero)aromatic systems, the rationale of CN⋯Br halogen bonding can also be employed in purely aliphatic systems.
中文翻译:

通过π堆积缩短CN⋯Br–Csp3卤素键
提出了2-(二溴甲基)苯甲腈的 X 射线结构,其特征是腈基团和 C(sp 3
![[三键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) ) 连接的溴原子之间具有出人意料的短 C N⋯Br 卤素键距离。尽管腈 N 原子的路易斯碱性质较弱且 Br 原子上不存在强吸电子基团,但 π 堆积显着增强了相互作用伙伴的电子供体和受体能力。因此,除了普通(杂)芳香族系统之外,C N⋯B r 卤素键合的基本原理也可以用于纯脂肪族系统。
) 连接的溴原子之间具有出人意料的短 C N⋯Br 卤素键距离。尽管腈 N 原子的路易斯碱性质较弱且 Br 原子上不存在强吸电子基团,但 π 堆积显着增强了相互作用伙伴的电子供体和受体能力。因此,除了普通(杂)芳香族系统之外,C N⋯B r 卤素键合的基本原理也可以用于纯脂肪族系统。



























 京公网安备 11010802027423号
京公网安备 11010802027423号