当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Equilibrium and Thermodynamic Analysis of Transition Metal Complexes Supported by Bis(imino)pyridine in Different Solvents
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.jced.4c00028 Xinyan Li 1 , Xiaoli Ma 1 , Wenyue Zhang 1 , Ziyuan Pang 1 , Cancan Yang 1 , Zhi Yang 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.jced.4c00028 Xinyan Li 1 , Xiaoli Ma 1 , Wenyue Zhang 1 , Ziyuan Pang 1 , Cancan Yang 1 , Zhi Yang 1
Affiliation
|
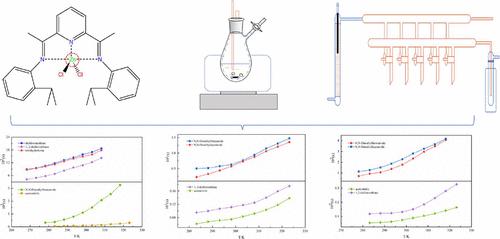
In this work, a static analysis method was used to measure the solubility data of L1 (1) (L1 = 2, 6-bis{1-[(2-isopropylphenyl)imino]ethyl}pyridine) in five pure solvents, and L1Zn (2) (L1Zn = [2, 6-bis{1-[(2-isopropylphenyl)imino]ethyl}pyridine]ZnCl2) and L1Mn (3) (L1Mn = [2, 6-bis{1-[(2-isopropylphenyl)imino]ethyl}pyridine]MnCl2) in four pure solvents. Based on the results of the experiment, it was concluded that the solubility of the three complexes also increased as the temperature increased. Eight thermodynamic models were used to correlate experimental solubility data. The results yielded a good fit with an average absolute relative deviation (ARD) of less than 3.5% and an average root mean square deviation (RMSD) of less than 0.15% for these models. In addition, the range of molecular electrostatic potentials of the three compounds was obtained by molecular electrostatic potential analysis. Reasons for solubility differences were analyzed by calculating the Hansen solubility parameters of L1. The solubility data measured in this paper can provide a theoretical basis and reference standard for the selection of reaction and recrystallization solvents in future experiments.
中文翻译:

不同溶剂中双(亚氨基)吡啶负载过渡金属配合物的固液平衡及热力学分析
本工作采用静态分析方法测量了L 1 (1) (L 1 = 2, 6-bis{1-[(2-异丙基苯基)亚氨基]乙基}吡啶)在五种纯溶剂中的溶解度数据,和L 1 Zn (2) (L 1 Zn = [2, 6-双{1-[(2-异丙基苯基)亚氨基]乙基}吡啶]ZnCl 2 )和L 1 Mn (3) (L 1 Mn = [2 ,6-双{1-[(2-异丙基苯基)亚氨基]乙基}吡啶]MnCl 2 )在四种纯溶剂中。根据实验结果,得出结论:三种配合物的溶解度也随着温度的升高而增加。使用八个热力学模型来关联实验溶解度数据。这些模型的结果拟合良好,平均绝对相对偏差 (ARD) 小于 3.5%,平均均方根偏差 (RMSD) 小于 0.15%。此外,通过分子静电势分析获得了三种化合物的分子静电势范围。通过计算L 1的Hansen溶解度参数分析了溶解度差异的原因。本文测得的溶解度数据可为今后实验中反应和重结晶溶剂的选择提供理论依据和参考标准。
更新日期:2024-03-28
中文翻译:

不同溶剂中双(亚氨基)吡啶负载过渡金属配合物的固液平衡及热力学分析
本工作采用静态分析方法测量了L 1 (1) (L 1 = 2, 6-bis{1-[(2-异丙基苯基)亚氨基]乙基}吡啶)在五种纯溶剂中的溶解度数据,和L 1 Zn (2) (L 1 Zn = [2, 6-双{1-[(2-异丙基苯基)亚氨基]乙基}吡啶]ZnCl 2 )和L 1 Mn (3) (L 1 Mn = [2 ,6-双{1-[(2-异丙基苯基)亚氨基]乙基}吡啶]MnCl 2 )在四种纯溶剂中。根据实验结果,得出结论:三种配合物的溶解度也随着温度的升高而增加。使用八个热力学模型来关联实验溶解度数据。这些模型的结果拟合良好,平均绝对相对偏差 (ARD) 小于 3.5%,平均均方根偏差 (RMSD) 小于 0.15%。此外,通过分子静电势分析获得了三种化合物的分子静电势范围。通过计算L 1的Hansen溶解度参数分析了溶解度差异的原因。本文测得的溶解度数据可为今后实验中反应和重结晶溶剂的选择提供理论依据和参考标准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号