Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Imparting As(III) Responsiveness to the Choline Response Transcriptional Regulator BetI
ACS Omega ( IF 4.1 ) Pub Date : 2024-03-26 , DOI: 10.1021/acsomega.3c09604 Ryo Yamaguchi 1 , Tetsuaki Yamamoto 1 , Daisuke Umeno 2 , Katsumasa Kamiya 3 , Shigeko Kawai-Noma 1
ACS Omega ( IF 4.1 ) Pub Date : 2024-03-26 , DOI: 10.1021/acsomega.3c09604 Ryo Yamaguchi 1 , Tetsuaki Yamamoto 1 , Daisuke Umeno 2 , Katsumasa Kamiya 3 , Shigeko Kawai-Noma 1
Affiliation
|
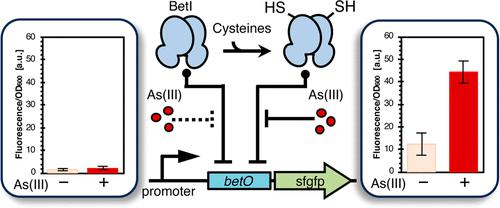
The development of a low-cost and user-friendly sensor using microorganisms to monitor the presence of As(III) on earth has garnered significant attention. In conventional research on microbial As(III) sensors, the focus has been on transcription factor ArsR, which plays a role in As(III) metabolism. However, we recently discovered that LuxR, a quorum-sensing control factor in Vibrio fischeri that contains multiple cysteine residues, acted as an As(III) sensor despite having no role in As(III) metabolism. This finding suggested that any protein could be an As(III) sensor if cysteine residues were incorporated. In this study, we aimed to confer As(III) responsiveness to BetI, a transcriptional repressor of the TetR family involved in osmotic regulation of the choline response, unrelated to As(III) metabolism. Based on the BetI structure constructed using molecular dynamics calculations, we generated a series of mutants in which each of the three amino acids not critical for function was substituted with cysteine. Subsequent examination of their response to As(III) revealed that the cysteine-substituted mutant, incorporating all three substitutions, demonstrated As(III) responsiveness. This was evidenced by the fluorescence intensity of the downstream reporter superfolder green fluorescent protein expression regulated by the operator region. Intriguingly, the BetI cysteine mutant maintained its binding responsiveness to the natural ligand choline. We successfully engineered an OR logic gate capable of responding to two orthogonal ligands using a single protein.
中文翻译:

赋予胆碱反应转录调节因子 BetI As(III) 反应性
使用微生物监测地球上 As(III) 存在的低成本且用户友好的传感器的开发引起了人们的广泛关注。在微生物 As(III) 传感器的常规研究中,重点是转录因子 ArsR,它在 As(III) 代谢中发挥作用。然而,我们最近发现 LuxR(费氏弧菌中的一种群体感应控制因子,含有多个半胱氨酸残基)尽管在 As(III) 代谢中没有作用,但可以充当 As(III) 传感器。这一发现表明,如果掺入半胱氨酸残基,任何蛋白质都可以成为 As(III) 传感器。在这项研究中,我们的目的是赋予 As(III) 对 BetI 的反应性,BetI 是 TetR 家族的转录抑制因子,参与胆碱反应的渗透调节,与 As(III) 代谢无关。基于使用分子动力学计算构建的 BetI 结构,我们生成了一系列突变体,其中对功能不重要的三个氨基酸中的每一个都被半胱氨酸取代。随后对它们对 As(III) 反应的检查表明,包含所有三种取代的半胱氨酸取代突变体表现出 As(III) 反应性。受操作区域调节的下游报告超级文件夹绿色荧光蛋白表达的荧光强度证明了这一点。有趣的是,BetI 半胱氨酸突变体保持了其对天然配体胆碱的结合反应性。我们成功地设计了一个“或”逻辑门,能够使用单个蛋白质响应两个正交配体。
更新日期:2024-03-26
中文翻译:

赋予胆碱反应转录调节因子 BetI As(III) 反应性
使用微生物监测地球上 As(III) 存在的低成本且用户友好的传感器的开发引起了人们的广泛关注。在微生物 As(III) 传感器的常规研究中,重点是转录因子 ArsR,它在 As(III) 代谢中发挥作用。然而,我们最近发现 LuxR(费氏弧菌中的一种群体感应控制因子,含有多个半胱氨酸残基)尽管在 As(III) 代谢中没有作用,但可以充当 As(III) 传感器。这一发现表明,如果掺入半胱氨酸残基,任何蛋白质都可以成为 As(III) 传感器。在这项研究中,我们的目的是赋予 As(III) 对 BetI 的反应性,BetI 是 TetR 家族的转录抑制因子,参与胆碱反应的渗透调节,与 As(III) 代谢无关。基于使用分子动力学计算构建的 BetI 结构,我们生成了一系列突变体,其中对功能不重要的三个氨基酸中的每一个都被半胱氨酸取代。随后对它们对 As(III) 反应的检查表明,包含所有三种取代的半胱氨酸取代突变体表现出 As(III) 反应性。受操作区域调节的下游报告超级文件夹绿色荧光蛋白表达的荧光强度证明了这一点。有趣的是,BetI 半胱氨酸突变体保持了其对天然配体胆碱的结合反应性。我们成功地设计了一个“或”逻辑门,能够使用单个蛋白质响应两个正交配体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号