当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PDA@UiO-66-NH2-derived nitrogen and oxygen-doped hierarchical porous carbon for efficient adsorption of BPA and dyes
Separation and Purification Technology ( IF 8.6 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.seppur.2024.127169 Xinyu Yuan , Xiaoyan Wang , Songqing Hu , Shuangqing Sun , Chunling Li
Separation and Purification Technology ( IF 8.6 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.seppur.2024.127169 Xinyu Yuan , Xiaoyan Wang , Songqing Hu , Shuangqing Sun , Chunling Li
|
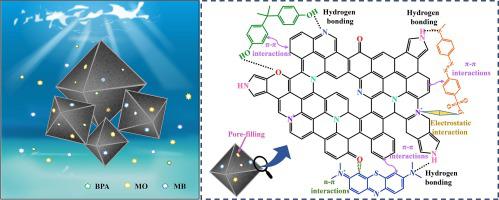
MOF-derived carbon materials have garnered significant attention in the field of adsorption due to their abundant porosity, excellent stability, and structural diversity. In this study, nitrogen and oxygen-doped hierarchical porous carbon materials were obtained by carbonizing PDA@UiO-66-NH at various temperatures and subsequently etching with hydrofluoric acid for different times. The adsorption capacity of these materials towards bisphenol a (BPA), methyl orange (MO), and methylene blue (MB) was evaluated. The results suggest excessive carbonization temperature and etching time may cause the collapse of the pore structure. In addition, the introduction of a PDA carbon layer can reduce the nitrogen–oxygen ratio and modify the nitrogen species in the materials. Among these materials, UPCH800, which was carbonized at 800 °C followed by etching for 6 h, exhibits the best adsorption performance towards BPA and MO, with a maximum adsorbed amount of 350.51 and 417.34 mg/g respectively. For MB, the maximum adsorption amount is 227.53 mg/g. All adsorptions reach equilibrium within 1 h. Thermodynamic studies reveal that the adsorption of MO by UPCH800 is an entropy-driven adsorption process, while the adsorption of BPA and MB is an enthalpy-driven exothermic process. Based on experimental and characterization results, the primary adsorption mechanisms involve π-π interactions, n-π interactions, pore-filling, and hydrogen bonding between the pollutants and the material. Moreover, UPCH800 exhibits high removal efficiency after 5 adsorption cycles, making it a promising material for the adsorption of BPA and MO.
中文翻译:

PDA@UiO-66-NH2 衍生的氮和氧掺杂的分级多孔碳可有效吸附 BPA 和染料
MOF衍生的碳材料由于其丰富的孔隙率、优异的稳定性和结构多样性而在吸附领域引起了广泛关注。本研究通过在不同温度下碳化PDA@UiO-66-NH并随后用氢氟酸蚀刻不同时间获得了氮和氧掺杂的分级多孔碳材料。评估了这些材料对双酚 A (BPA)、甲基橙 (MO) 和亚甲基蓝 (MB) 的吸附能力。结果表明,过高的碳化温度和蚀刻时间可能会导致孔隙结构的崩溃。此外,引入PDA碳层可以降低氮氧比并改变材料中的氮物种。其中,经过800℃碳化并蚀刻6h的UPCH800对BPA和MO表现出最好的吸附性能,最大吸附量分别为350.51和417.34 mg/g。对于MB,最大吸附量为227.53 mg/g。所有吸附在 1 小时内达到平衡。热力学研究表明UPCH800对MO的吸附是熵驱动的吸附过程,而BPA和MB的吸附是焓驱动的放热过程。根据实验和表征结果,主要吸附机制包括污染物与材料之间的π-π相互作用、n-π相互作用、孔隙填充以及氢键作用。此外,UPCH800在5次吸附循环后表现出较高的去除效率,使其成为吸附BPA和MO的有前途的材料。
更新日期:2024-03-19
中文翻译:

PDA@UiO-66-NH2 衍生的氮和氧掺杂的分级多孔碳可有效吸附 BPA 和染料
MOF衍生的碳材料由于其丰富的孔隙率、优异的稳定性和结构多样性而在吸附领域引起了广泛关注。本研究通过在不同温度下碳化PDA@UiO-66-NH并随后用氢氟酸蚀刻不同时间获得了氮和氧掺杂的分级多孔碳材料。评估了这些材料对双酚 A (BPA)、甲基橙 (MO) 和亚甲基蓝 (MB) 的吸附能力。结果表明,过高的碳化温度和蚀刻时间可能会导致孔隙结构的崩溃。此外,引入PDA碳层可以降低氮氧比并改变材料中的氮物种。其中,经过800℃碳化并蚀刻6h的UPCH800对BPA和MO表现出最好的吸附性能,最大吸附量分别为350.51和417.34 mg/g。对于MB,最大吸附量为227.53 mg/g。所有吸附在 1 小时内达到平衡。热力学研究表明UPCH800对MO的吸附是熵驱动的吸附过程,而BPA和MB的吸附是焓驱动的放热过程。根据实验和表征结果,主要吸附机制包括污染物与材料之间的π-π相互作用、n-π相互作用、孔隙填充以及氢键作用。此外,UPCH800在5次吸附循环后表现出较高的去除效率,使其成为吸附BPA和MO的有前途的材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号