当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reconfiguration of the charge density difference of nitrogen-doped graphene by covalently bonded Cu-N4 active sites boosting thermodynamics and performance in aprotic Li-CO2 battery
Energy Storage Materials ( IF 20.4 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.ensm.2024.103354 Yunyun Xu , Xijuan Li , Yuejiao Li , Yi Wang , Li Song , Junchao Ding , Xiaoli Fan , Jianping He , Tao Wang , Zhong-Shuai Wu
Energy Storage Materials ( IF 20.4 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.ensm.2024.103354 Yunyun Xu , Xijuan Li , Yuejiao Li , Yi Wang , Li Song , Junchao Ding , Xiaoli Fan , Jianping He , Tao Wang , Zhong-Shuai Wu
|
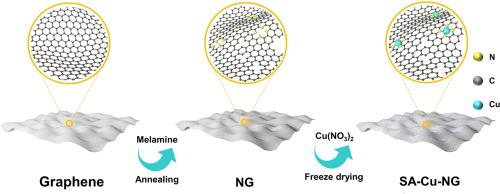
The slow kinetics of the CO reduction and evolution reactions in the Li-CO battery result in a high overpotential, low energy efficiency and undesired life. Exploring the durable electrocatalysts with high activity for CO reduction and evolution processes in aprotic Li-CO batteries is of great significance for CO capture and utilization. Herein, single-atom copper uniformly anchored on nitrogen-doped graphene (SA-Cu-NG) was demonstrated as a durable catalyst for the rechargeable Li-CO battery. The resulting Li-CO battery shows a remarkable specific capacity of 29033 mAh g at 100 mA g, an ultra-long life up to 538 cycles (over 2730 h), and a low overpotential of 1.47 V (1000 mA g), outperforming the reported Li-CO batteries. The X-ray absorption fine structure analysis of SA-Cu-NG unravels that the covalent effect between Cu and N, which exists in the form of Cu-N in nitrogen-doped graphene. Further, it is theoretically elucidated that the covalent effect of Cu-N leads to the reconfiguration of the charge density difference on nitrogen-doped graphene, thereby improving the adsorption of CO and weakening the decomposition barrier of the discharge products on the surface single-atom copper, thus optimizing the nucleation decomposition process. In conclusion, the exceptional performances of Li-CO battery are attributed to the superior catalytic activity on Cu-N sites and the excellent electronic conductivity of nitrogen-doped graphene, activating the reversible process of discharge product formation and decomposition.
中文翻译:

通过共价键合的 Cu-N4 活性位点重新配置氮掺杂石墨烯的电荷密度差,提高非质子 Li-CO2 电池的热力学和性能
Li-CO电池中CO还原和析出反应的缓慢动力学导致高过电势、低能量效率和不理想的寿命。探索用于非质子Li-CO电池中CO还原和析出过程的高活性耐用电催化剂对于CO捕获和利用具有重要意义。在此,均匀锚定在氮掺杂石墨烯上的单原子铜(SA-Cu-NG)被证明是可充电锂二氧化碳电池的耐用催化剂。由此产生的 Li-CO 电池在 100 mA g-1 时表现出 29033 mAh g-1 的非凡比容量、高达 538 次循环(超过 2730 小时)的超长寿命以及 1.47 V(1000 mA g-1)的低过电势,优于报道了锂二氧化碳电池。 SA-Cu-NG的X射线吸收精细结构分析揭示了Cu和N之间的共价效应,在氮掺杂石墨烯中以Cu-N的形式存在。进一步从理论上阐明了Cu-N的共价效应导致氮掺杂石墨烯上电荷密度差的重构,从而提高了CO的吸附并削弱了放电产物在表面单原子上的分解势垒。铜,从而优化成核分解过程。总之,Li-CO电池的优异性能归因于Cu-N位点上优异的催化活性和氮掺杂石墨烯优异的电子导电性,激活了放电产物形成和分解的可逆过程。
更新日期:2024-03-18
中文翻译:

通过共价键合的 Cu-N4 活性位点重新配置氮掺杂石墨烯的电荷密度差,提高非质子 Li-CO2 电池的热力学和性能
Li-CO电池中CO还原和析出反应的缓慢动力学导致高过电势、低能量效率和不理想的寿命。探索用于非质子Li-CO电池中CO还原和析出过程的高活性耐用电催化剂对于CO捕获和利用具有重要意义。在此,均匀锚定在氮掺杂石墨烯上的单原子铜(SA-Cu-NG)被证明是可充电锂二氧化碳电池的耐用催化剂。由此产生的 Li-CO 电池在 100 mA g-1 时表现出 29033 mAh g-1 的非凡比容量、高达 538 次循环(超过 2730 小时)的超长寿命以及 1.47 V(1000 mA g-1)的低过电势,优于报道了锂二氧化碳电池。 SA-Cu-NG的X射线吸收精细结构分析揭示了Cu和N之间的共价效应,在氮掺杂石墨烯中以Cu-N的形式存在。进一步从理论上阐明了Cu-N的共价效应导致氮掺杂石墨烯上电荷密度差的重构,从而提高了CO的吸附并削弱了放电产物在表面单原子上的分解势垒。铜,从而优化成核分解过程。总之,Li-CO电池的优异性能归因于Cu-N位点上优异的催化活性和氮掺杂石墨烯优异的电子导电性,激活了放电产物形成和分解的可逆过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号