当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of reaction media on hydrogenolysis of polyethylene plastic waste: Polymer-surface interactions in small alkane/polymer blends
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.apcatb.2024.123969 Mehdi Zare , Pavel A. Kots , Zachary R. Hinton , Thomas H. Epps , LaShanda T.J. Korley , Stavros Caratzoulas , Dionisios G. Vlachos
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.apcatb.2024.123969 Mehdi Zare , Pavel A. Kots , Zachary R. Hinton , Thomas H. Epps , LaShanda T.J. Korley , Stavros Caratzoulas , Dionisios G. Vlachos
|
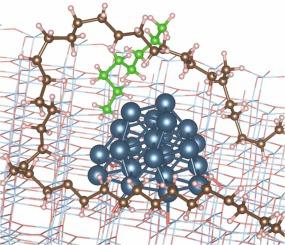
The polymer reaction media and its properties can be altered by recycling a fraction of liquid products or adding alkane solvents. Less clear is whether this strategy affects hydrogenolysis. Herein, we investigated the effect of short-chain alkanes C consisting of n carbons (n=8, 16, and 32) on the upcycling of high-density polyethylene (HDPE) plastic waste to lubricant-range products over Ru/TiO catalysts by multiscale simulations and experiments. First, we trained a force field for polymer/surface interactions on a Ru nanoparticle (NP) supported on TiO. Using replica exchange molecular dynamics simulations, we studied the effect of small hydrocarbons on the adsorption of a surrogate polymer, C, on the catalyst. We found segregation of long chains (C) at the catalyst surface due to the enthalpy gained by adsorbing more C-C bonds of the long chains, compensating for entropic losses upon adsorption. Short-chain molecules decrease the adsorbed carbons of long chains on the Ru NP due to blocking Ru active sites. Compared to the bulk chains, competitive adsorption results in a broader, heavy-tailed distribution of end-to-end distance of adsorbed chains. Our experiments demonstrated that catalyst activity declines significantly beyond simple dilution due to changes in polymer adsorption, and tuning the reaction media by creating suitable blends impacts hydrogenolysis. Density distributions for a 50:50%wt mixture of PP and PE show that PE chains are segregated at the surface, so they are prone to C-C bond breaking much faster than PP chains. H/D exchange experiments show preferential deuteration of PE, while CH groups of PP remain undeuterated. This may be explained by the preferential sorption of PE over PP, leading to specific distribution in the polymer blend.
中文翻译:

反应介质对聚乙烯塑料废料氢解的影响:小烷烃/聚合物共混物中聚合物-表面相互作用
聚合物反应介质及其性质可以通过回收一部分液体产物或添加烷烃溶剂来改变。不太清楚的是这种策略是否会影响氢解作用。在此,我们研究了由 n 个碳(n=8、16 和 32)组成的短链烷烃 C 在 Ru/TiO 催化剂上将高密度聚乙烯 (HDPE) 塑料废物升级为润滑油系列产品的影响:多尺度模拟和实验。首先,我们在 TiO2 支撑的 Ru 纳米颗粒 (NP) 上训练了聚合物/表面相互作用的力场。利用复制交换分子动力学模拟,我们研究了小分子碳氢化合物对替代聚合物 C 在催化剂上吸附的影响。我们发现长链 (C) 在催化剂表面发生偏析,这是由于通过吸附更多长链的 CC 键而获得的焓,从而补偿了吸附时的熵损失。由于阻断 Ru 活性位点,短链分子减少了 Ru NP 上长链的吸附碳。与本体链相比,竞争吸附导致吸附链的端到端距离具有更宽的重尾分布。我们的实验表明,由于聚合物吸附的变化,催化剂活性比简单稀释时显着下降,并且通过创建合适的混合物来调整反应介质会影响氢解。 PP 和 PE 50:50%wt 混合物的密度分布表明,PE 链在表面偏析,因此它们比 PP 链更容易发生 CC 键断裂。 H/D交换实验表明PE优先氘化,而PP的CH基团保持未氘化。这可以通过 PE 相对于 PP 的优先吸附来解释,从而导致聚合物共混物中的特定分布。
更新日期:2024-03-19
中文翻译:

反应介质对聚乙烯塑料废料氢解的影响:小烷烃/聚合物共混物中聚合物-表面相互作用
聚合物反应介质及其性质可以通过回收一部分液体产物或添加烷烃溶剂来改变。不太清楚的是这种策略是否会影响氢解作用。在此,我们研究了由 n 个碳(n=8、16 和 32)组成的短链烷烃 C 在 Ru/TiO 催化剂上将高密度聚乙烯 (HDPE) 塑料废物升级为润滑油系列产品的影响:多尺度模拟和实验。首先,我们在 TiO2 支撑的 Ru 纳米颗粒 (NP) 上训练了聚合物/表面相互作用的力场。利用复制交换分子动力学模拟,我们研究了小分子碳氢化合物对替代聚合物 C 在催化剂上吸附的影响。我们发现长链 (C) 在催化剂表面发生偏析,这是由于通过吸附更多长链的 CC 键而获得的焓,从而补偿了吸附时的熵损失。由于阻断 Ru 活性位点,短链分子减少了 Ru NP 上长链的吸附碳。与本体链相比,竞争吸附导致吸附链的端到端距离具有更宽的重尾分布。我们的实验表明,由于聚合物吸附的变化,催化剂活性比简单稀释时显着下降,并且通过创建合适的混合物来调整反应介质会影响氢解。 PP 和 PE 50:50%wt 混合物的密度分布表明,PE 链在表面偏析,因此它们比 PP 链更容易发生 CC 键断裂。 H/D交换实验表明PE优先氘化,而PP的CH基团保持未氘化。这可以通过 PE 相对于 PP 的优先吸附来解释,从而导致聚合物共混物中的特定分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号