当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling the decomposing and mineralizing mechanism of novel perfluoroalkyl acids via hydroxyl radical dominated electrochemical oxidation
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.apcatb.2024.123983 Meng Li , Hongguo Zhang , Jiliang Cheng , Jiayu Song , Wei Han , Shaoqi Zhou , Zhao-Xin Zhang , Qiong Wu , King Lun Yeung , Ce-Hui Mo
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.apcatb.2024.123983 Meng Li , Hongguo Zhang , Jiliang Cheng , Jiayu Song , Wei Han , Shaoqi Zhou , Zhao-Xin Zhang , Qiong Wu , King Lun Yeung , Ce-Hui Mo
|
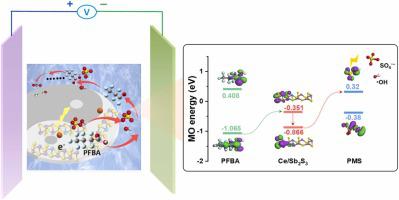
A cerium-doped SbS anode is successfully fabricated for perfluoroalkyl acids (PFAAs) degradation via a hydroxyl radical dominant electrochemical oxidation (EO) coupled peroxymonosulfate (PMS) reaction. Various PFAAs can be efficiently removed within 25 min with high mineralization and low energy consumption. The hydroxyl radical, sulfate radical, and electron transfer are responsible for PFAAs degradation, and the hydroxyl radical played a dominant role. The electron can be moved from the highest occupied molecular orbital of these PFAAs to the lowest unoccupied molecular orbital of PMS via potential energy difference, further decomposing PMS to generate hydroxyl and sulfate radical for PFAAs degradation. Besides, the corresponding degradation pathway, defluorination, and Gibbs free energy of the reaction for each PFAAs were elucidated based on the identified intermediates and density functional theory calculations. The current study provides insight into the efficient decomposition mechanism of various PFAAs during EO and highlights its promising potential in environmental remediation.
中文翻译:

通过羟基自由基主导的电化学氧化揭示新型全氟烷基酸的分解和矿化机制
成功制备了掺铈 SbS 阳极,通过羟基自由基主导的电化学氧化 (EO) 耦合过一硫酸盐 (PMS) 反应降解全氟烷基酸 (PFAA)。各种PFAA可在25分钟内高效去除,矿化度高,能耗低。羟基自由基、硫酸根和电子转移是PFAA降解的原因,其中羟基自由基起主导作用。通过势能差,电子可以从这些PFAA的最高占据分子轨道移动到PMS的最低未占据分子轨道,进一步分解PMS,产生羟基和硫酸根,从而降解PFAA。此外,基于确定的中间体和密度泛函理论计算,阐明了每种PFAA的相应降解途径、脱氟反应和吉布斯自由能。目前的研究深入了解了EO过程中各种PFAA的有效分解机制,并强调了其在环境修复中的巨大潜力。
更新日期:2024-03-19
中文翻译:

通过羟基自由基主导的电化学氧化揭示新型全氟烷基酸的分解和矿化机制
成功制备了掺铈 SbS 阳极,通过羟基自由基主导的电化学氧化 (EO) 耦合过一硫酸盐 (PMS) 反应降解全氟烷基酸 (PFAA)。各种PFAA可在25分钟内高效去除,矿化度高,能耗低。羟基自由基、硫酸根和电子转移是PFAA降解的原因,其中羟基自由基起主导作用。通过势能差,电子可以从这些PFAA的最高占据分子轨道移动到PMS的最低未占据分子轨道,进一步分解PMS,产生羟基和硫酸根,从而降解PFAA。此外,基于确定的中间体和密度泛函理论计算,阐明了每种PFAA的相应降解途径、脱氟反应和吉布斯自由能。目前的研究深入了解了EO过程中各种PFAA的有效分解机制,并强调了其在环境修复中的巨大潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号