当前位置:
X-MOL 学术
›
Nano Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of label-free triboelectric nanosensors as screening platforms for anti-tumor drugs
Nano Energy ( IF 17.6 ) Pub Date : 2024-03-17 , DOI: 10.1016/j.nanoen.2024.109519 Yu-Ying Cheng , Anindita Ganguly , Yi-Yun Cheng , Christopher Llynard D. Ortiz , Arnab Pal , Pramod Shah , Kuldeep Kaswan , Lee-Wei Yang , Zong-Hong Lin
Nano Energy ( IF 17.6 ) Pub Date : 2024-03-17 , DOI: 10.1016/j.nanoen.2024.109519 Yu-Ying Cheng , Anindita Ganguly , Yi-Yun Cheng , Christopher Llynard D. Ortiz , Arnab Pal , Pramod Shah , Kuldeep Kaswan , Lee-Wei Yang , Zong-Hong Lin
|
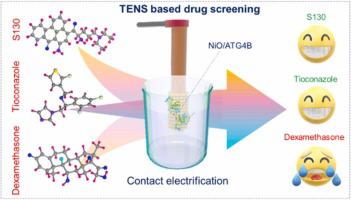
This study introduces a general label-free drug screening platform with potential to support high-throughput drug screening for any protein target. Its innovation lies in quantifying the affinity of molecule-molecule interactions via voltage output variation, which achieves unprecedented selectivity and sensitivity. It combines computational analysis complemented by experimental substantiation with a solid-liquid triboelectric nanosensor. Owing to its high binding affinity, FKBP-rapamycin is used as a model system to demonstrate the platform's sensitivity. Subsequently, the research is extended to study drug interactions with an oncogenic protein ATG4B. The findings unveil the binding of S130 and Tioconazole to ATG4B, a critical cysteine protease involved in autophagy, while revealing that Dexamethasone does not bind ATG4B. This binding exerts a specific inhibitory effect on autophagic flux and triggers cancer cell apoptosis. The results support the previously verified inhibitory effects of these drugs and the effectiveness of a newly developed self-powered drug screening platform, leveraging the principles of solid-liquid contact electrification. This approach adeptly confronts enduring challenges in traditional drug development methods where biochemical assays need to be designed for each individual protein target or only time-consuming and concentration-demanding molecular interaction measurements were made available.
中文翻译:

开发无标记摩擦纳米传感器作为抗肿瘤药物筛选平台
这项研究介绍了一个通用的无标记药物筛选平台,有可能支持任何蛋白质靶标的高通量药物筛选。其创新之处在于通过电压输出变化来量化分子间相互作用的亲和力,从而实现了前所未有的选择性和灵敏度。它将计算分析与固液摩擦纳米传感器的实验证实相结合。由于其高结合亲和力,FKBP-雷帕霉素被用作模型系统来证明该平台的敏感性。随后,该研究扩展到研究药物与致癌蛋白 ATG4B 的相互作用。研究结果揭示了 S130 和噻康唑与 ATG4B(一种参与自噬的关键半胱氨酸蛋白酶)的结合,同时揭示了地塞米松不结合 ATG4B。这种结合对自噬流产生特异性抑制作用并引发癌细胞凋亡。结果支持了先前验证的这些药物的抑制作用以及新开发的自供电药物筛选平台的有效性,该平台利用固液接触电化原理。这种方法巧妙地应对了传统药物开发方法中持久的挑战,在传统药物开发方法中,需要针对每个单独的蛋白质靶点设计生化测定,或者仅提供耗时且高浓度的分子相互作用测量。
更新日期:2024-03-17
中文翻译:

开发无标记摩擦纳米传感器作为抗肿瘤药物筛选平台
这项研究介绍了一个通用的无标记药物筛选平台,有可能支持任何蛋白质靶标的高通量药物筛选。其创新之处在于通过电压输出变化来量化分子间相互作用的亲和力,从而实现了前所未有的选择性和灵敏度。它将计算分析与固液摩擦纳米传感器的实验证实相结合。由于其高结合亲和力,FKBP-雷帕霉素被用作模型系统来证明该平台的敏感性。随后,该研究扩展到研究药物与致癌蛋白 ATG4B 的相互作用。研究结果揭示了 S130 和噻康唑与 ATG4B(一种参与自噬的关键半胱氨酸蛋白酶)的结合,同时揭示了地塞米松不结合 ATG4B。这种结合对自噬流产生特异性抑制作用并引发癌细胞凋亡。结果支持了先前验证的这些药物的抑制作用以及新开发的自供电药物筛选平台的有效性,该平台利用固液接触电化原理。这种方法巧妙地应对了传统药物开发方法中持久的挑战,在传统药物开发方法中,需要针对每个单独的蛋白质靶点设计生化测定,或者仅提供耗时且高浓度的分子相互作用测量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号