当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring novel pyrazole-nitroimidazole hybrids: Synthesis and antiprotozoal activity against the human pathogen trichomonas vaginalis
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.bmc.2024.117679 Rafaela Corrêa Silva 1 , Anna De Freitas 2 , Bruno Vicente 3 , Victor Midlej 4 , Maurício Silva Dos Santos 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.bmc.2024.117679 Rafaela Corrêa Silva 1 , Anna De Freitas 2 , Bruno Vicente 3 , Victor Midlej 4 , Maurício Silva Dos Santos 1
Affiliation
|
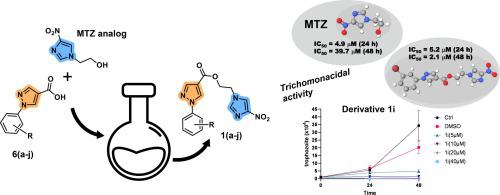
Trichomoniasis, a prevalent sexually transmitted infection (STI) caused by the protozoan , has gained increased significance globally. Its relevance has grown in recent years due to its association with a heightened risk of acquiring and transmitting the human immunodeficiency virus (HIV) and other STIs. In addition, many publications have revealed a potential link between trichomoniasis and certain cancers. Metronidazole (MTZ), a nitroimidazole compound developed over 50 years ago, remains the first-choice drug for treatment. However, reports of genotoxicity and side effects underscore the necessity for new compounds to address this pressing global health concern. In this study, we synthesized ten pyrazole-nitroimidazoles and 4-nitro-1-(hydroxyethyl)-1-imidazole , an analog of metronidazole (MTZ), and assessed their trichomonacidal and cytotoxic effects. All compounds and exhibited IC values ≤ 20 μM and ≤ 41 μM, after 24 h and 48 h, respectively. Compounds (IC 5.3 μM), (IC 4.8 μM), and (IC 5.2 μM) exhibited potencies equivalent to MTZ (IC 4.9 μM), the reference drug, after 24 h. Notably, compound showed high anti-trichomonas activity after 24 h (IC 5.2 μM) and 48 h (IC 2.1 μM). Additionally, all compounds demonstrated either non-cytotoxic to HeLa cells (CC > 100 μM) or low cytotoxicity (CC between 69 and 100 μM). These findings suggest that pyrazole-nitroimidazole derivatives represent a promising heterocyclic system, serving as a potential lead for further optimization in trichomoniasis chemotherapy.
中文翻译:

探索新型吡唑-硝基咪唑杂化物:针对人类病原体阴道毛滴虫的合成和抗原虫活性
滴虫病是一种由原生动物引起的流行性传播感染 (STI),在全球范围内的重要性日益凸显。近年来,由于它与感染和传播人类免疫缺陷病毒(HIV)和其他性传播感染的风险增加有关,其重要性不断增强。此外,许多出版物揭示了滴虫病和某些癌症之间的潜在联系。甲硝唑 (MTZ) 是一种 50 多年前开发的硝基咪唑化合物,仍然是治疗的首选药物。然而,遗传毒性和副作用的报告强调了新化合物解决这一紧迫的全球健康问题的必要性。在本研究中,我们合成了十种吡唑-硝基咪唑和4-硝基-1-(羟乙基)-1-咪唑(甲硝唑(MTZ)的类似物),并评估了它们的滴虫酸和细胞毒性作用。 24 小时和 48 小时后,所有化合物 和 的 IC 值分别≤ 20 μM 和 ≤ 41 μM。 24 小时后,化合物 (IC 5.3 μM)、(IC 4.8 μM) 和 (IC 5.2 μM) 表现出与参比药物 MTZ (IC 4.9 μM) 相当的效力。值得注意的是,化合物在 24 小时(IC 5.2 μM)和 48 小时(IC 2.1 μM)后显示出高抗滴虫活性。此外,所有化合物均表现出对 HeLa 细胞无细胞毒性(CC > 100 μM)或低细胞毒性(CC 在 69 至 100 μM 之间)。这些发现表明,吡唑-硝基咪唑衍生物代表了一种有前途的杂环系统,可作为进一步优化滴虫化疗的潜在先导。
更新日期:2024-03-07
中文翻译:

探索新型吡唑-硝基咪唑杂化物:针对人类病原体阴道毛滴虫的合成和抗原虫活性
滴虫病是一种由原生动物引起的流行性传播感染 (STI),在全球范围内的重要性日益凸显。近年来,由于它与感染和传播人类免疫缺陷病毒(HIV)和其他性传播感染的风险增加有关,其重要性不断增强。此外,许多出版物揭示了滴虫病和某些癌症之间的潜在联系。甲硝唑 (MTZ) 是一种 50 多年前开发的硝基咪唑化合物,仍然是治疗的首选药物。然而,遗传毒性和副作用的报告强调了新化合物解决这一紧迫的全球健康问题的必要性。在本研究中,我们合成了十种吡唑-硝基咪唑和4-硝基-1-(羟乙基)-1-咪唑(甲硝唑(MTZ)的类似物),并评估了它们的滴虫酸和细胞毒性作用。 24 小时和 48 小时后,所有化合物 和 的 IC 值分别≤ 20 μM 和 ≤ 41 μM。 24 小时后,化合物 (IC 5.3 μM)、(IC 4.8 μM) 和 (IC 5.2 μM) 表现出与参比药物 MTZ (IC 4.9 μM) 相当的效力。值得注意的是,化合物在 24 小时(IC 5.2 μM)和 48 小时(IC 2.1 μM)后显示出高抗滴虫活性。此外,所有化合物均表现出对 HeLa 细胞无细胞毒性(CC > 100 μM)或低细胞毒性(CC 在 69 至 100 μM 之间)。这些发现表明,吡唑-硝基咪唑衍生物代表了一种有前途的杂环系统,可作为进一步优化滴虫化疗的潜在先导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号