当前位置:
X-MOL 学术
›
Comput. Biol. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Blood flow and emboli transport patterns during venoarterial extracorporeal membrane oxygenation: A computational fluid dynamics study
Computers in Biology and Medicine ( IF 7.7 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.compbiomed.2024.108263 Mehrdad Khamooshi , Avishka Wickramarachchi , Tim Byrne , Michael Seman , David F. Fletcher , Aidan Burrell , Shaun D. Gregory
Computers in Biology and Medicine ( IF 7.7 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.compbiomed.2024.108263 Mehrdad Khamooshi , Avishka Wickramarachchi , Tim Byrne , Michael Seman , David F. Fletcher , Aidan Burrell , Shaun D. Gregory
|
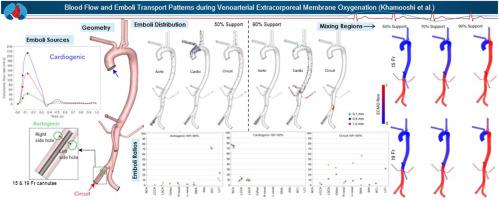
Despite advances in Venoarterial Extracorporeal Membrane Oxygenation (VA-ECMO), a significant mortality rate persists due to complications. The non-physiological blood flow dynamics of VA-ECMO may lead to neurological complications and organ ischemia. Continuous retrograde high-flow oxygenated blood enters through a return cannula placed in the femoral artery which opposes the pulsatile deoxygenated blood ejected by the left ventricle (LV), which impacts upper body oxygenation and subsequent hyperoxemia. The complications underscore the critical need to comprehend the impact of VA-ECMO support level and return cannula size, as mortality remains a significant concern. The aim of this study is to predict and provide insights into the complications associated with VA-ECMO using computational fluid dynamics (CFD) simulations. These complications will be assessed by characterising blood flow and emboli transport patterns through a comprehensive analysis of the influence of VA-ECMO support levels and arterial return cannula sizes. Patient-specific 3D aortic and major branch models, derived from a male patient's CT scan during VA-ECMO undergoing respiratory dysfunction, were analyzed using CFD. The investigation employed species transport and discrete particle tracking models to study ECMO blood (oxygenated) mixing with LV blood (deoxygenated) and to trace emboli transport patterns from potential sources (circuit, LV, and aorta wall). Two cannula sizes (15 Fr and 19 Fr) were tested alongside varying ECMO pump flow rates (50%, 70%, and 90% of the total cardiac output). Cannula size did not significantly affect oxygen transport. At 90% VA-ECMO support, all arteries distal to the aortic arch achieved 100% oxygen saturation. As support level decreased, oxygen transport to the upper body also decreased to a minimum saturation of 73%. Emboli transport varied substantially between emboli origin and VAECMO support level, with the highest risk of cerebral emboli coming from the LV with a 15 Fr cannula at 90% support. Arterial return cannula sizing minimally impacted blood oxygen distribution; however, it did influence the distribution of emboli released from the circuit and aortic wall. Notably, it was the support level alone that significantly affected the mixing zone of VA-ECMO and cardiac blood, subsequently influencing the risk of embolization of the cardiogenic source and oxygenation levels across various arterial branches.
中文翻译:

静脉动脉体外膜氧合过程中的血流和栓子转运模式:计算流体动力学研究
尽管静脉动脉体外膜氧合(VA-ECMO)取得了进展,但由于并发症,死亡率仍然很高。 VA-ECMO的非生理性血流动力学可能导致神经并发症和器官缺血。持续逆行的高流量含氧血液通过放置在股动脉中的回流插管进入,该回流插管与左心室(LV)喷射的脉动脱氧血液相反,从而影响上身氧合和随后的高氧血症。这些并发症强调了了解 VA-ECMO 支持水平和回流插管尺寸的影响的迫切需要,因为死亡率仍然是一个重大问题。本研究的目的是使用计算流体动力学 (CFD) 模拟来预测并深入了解 VA-ECMO 相关的并发症。这些并发症将通过综合分析 VA-ECMO 支持水平和动脉回流插管尺寸的影响来表征血流和栓子转运模式。使用 CFD 分析患者特异性 3D 主动脉和主要分支模型,该模型源自一名患有呼吸功能障碍的男性患者在 VA-ECMO 期间进行的 CT 扫描。该研究采用物种转运和离散粒子追踪模型来研究 ECMO 血液(含氧)与左心室血液(脱氧)的混合,并追踪潜在来源(回路、左心室和主动脉壁)的栓塞转运模式。测试了两种插管尺寸(15 Fr 和 19 Fr)以及不同的 ECMO 泵流量(总心输出量的 50%、70% 和 90%)。插管尺寸对氧气输送没有显着影响。在 90% VA-ECMO 支持下,主动脉弓远端的所有动脉均达到 100% 氧饱和度。随着支持水平的降低,输送到上半身的氧气也降低到最低饱和度 73%。栓塞来源和 VAECMO 支持水平之间的栓塞转运差异很大,其中脑栓塞的风险最高来自 90% 支持的 15 Fr 插管的左心室。动脉回流插管尺寸对血氧分布的影响最小;然而,它确实影响了从环路和主动脉壁释放的栓子的分布。值得注意的是,仅支持水平就显着影响了 VA-ECMO 和心脏血液的混合区,从而影响了心源性栓塞的风险和各动脉分支的氧合水平。
更新日期:2024-03-11
中文翻译:

静脉动脉体外膜氧合过程中的血流和栓子转运模式:计算流体动力学研究
尽管静脉动脉体外膜氧合(VA-ECMO)取得了进展,但由于并发症,死亡率仍然很高。 VA-ECMO的非生理性血流动力学可能导致神经并发症和器官缺血。持续逆行的高流量含氧血液通过放置在股动脉中的回流插管进入,该回流插管与左心室(LV)喷射的脉动脱氧血液相反,从而影响上身氧合和随后的高氧血症。这些并发症强调了了解 VA-ECMO 支持水平和回流插管尺寸的影响的迫切需要,因为死亡率仍然是一个重大问题。本研究的目的是使用计算流体动力学 (CFD) 模拟来预测并深入了解 VA-ECMO 相关的并发症。这些并发症将通过综合分析 VA-ECMO 支持水平和动脉回流插管尺寸的影响来表征血流和栓子转运模式。使用 CFD 分析患者特异性 3D 主动脉和主要分支模型,该模型源自一名患有呼吸功能障碍的男性患者在 VA-ECMO 期间进行的 CT 扫描。该研究采用物种转运和离散粒子追踪模型来研究 ECMO 血液(含氧)与左心室血液(脱氧)的混合,并追踪潜在来源(回路、左心室和主动脉壁)的栓塞转运模式。测试了两种插管尺寸(15 Fr 和 19 Fr)以及不同的 ECMO 泵流量(总心输出量的 50%、70% 和 90%)。插管尺寸对氧气输送没有显着影响。在 90% VA-ECMO 支持下,主动脉弓远端的所有动脉均达到 100% 氧饱和度。随着支持水平的降低,输送到上半身的氧气也降低到最低饱和度 73%。栓塞来源和 VAECMO 支持水平之间的栓塞转运差异很大,其中脑栓塞的风险最高来自 90% 支持的 15 Fr 插管的左心室。动脉回流插管尺寸对血氧分布的影响最小;然而,它确实影响了从环路和主动脉壁释放的栓子的分布。值得注意的是,仅支持水平就显着影响了 VA-ECMO 和心脏血液的混合区,从而影响了心源性栓塞的风险和各动脉分支的氧合水平。



























 京公网安备 11010802027423号
京公网安备 11010802027423号