当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Light-Switchable N-Alkylation Using Amine-Functionalized MOF
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2024-03-09 , DOI: 10.1016/j.apcatb.2024.123924 Yu Huang , Yaru Li , Dongsheng Zhang , Yuanqiang Mai , Flemming Besenbacher , Chuan Dong , Federico Rosei , Yong Yang , Yongwang Li , Hans Niemantsverdriet , Wenting Liang , Ren Su
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2024-03-09 , DOI: 10.1016/j.apcatb.2024.123924 Yu Huang , Yaru Li , Dongsheng Zhang , Yuanqiang Mai , Flemming Besenbacher , Chuan Dong , Federico Rosei , Yong Yang , Yongwang Li , Hans Niemantsverdriet , Wenting Liang , Ren Su
|
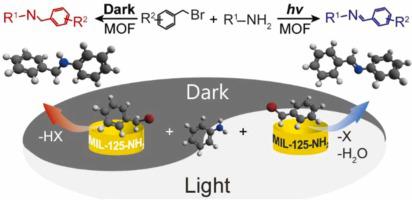
Catalytic N-alkylation is a frequently employed method to synthesize secondary amines and imines, yet selectivity control remains as a challenge that normally requires specialized catalysts under harsh reaction conditions. Here we propose a light-switchable N-alkylation of amines with aromatic halides for selective synthesis of secondary amines and imines, using an amine-functionalized metal-organic framework (MIL-125-NH) under mild conditions. The MIL-125-NH catalyst possesses Lewis acidic sites, which catalyze direct dehalogenative condensation of bromides with primary amines to produce secondary amines in the dark. Upon irradiation, the MIL-125-NH reduces molecular oxygen to create oxygen radicals, converting bromides into the corresponding aldehydes to yield imines a dehydrative coupling with amines. With appropriate acidity, rapid oxygen reduction kinetics, and optimized adsorption of aromatic bromides and generated water, the system catalyzes the conversion of a wide range of substrates, thus featuring it a promising method for applications.
中文翻译:

使用胺官能化 MOF 进行光开关 N-烷基化
催化 N-烷基化是合成仲胺和亚胺的常用方法,但选择性控制仍然是一个挑战,通常需要在恶劣的反应条件下使用专门的催化剂。在这里,我们提出了在温和条件下使用胺官能化金属有机框架(MIL-125-NH),用芳香族卤化物对胺进行光可切换的N-烷基化,以选择性合成仲胺和亚胺。 MIL-125-NH催化剂具有路易斯酸性位点,可催化溴化物与伯胺在暗处直接脱卤缩合生成仲胺。辐射后,MIL-125-NH 会还原分子氧,产生氧自由基,将溴化物转化为相应的醛,产生亚胺,与胺发生脱水偶联。该系统具有适当的酸度、快速的氧还原动力学以及对芳香族溴化物和生成水的优化吸附,可催化多种底物的转化,因此是一种有前景的应用方法。
更新日期:2024-03-09
中文翻译:

使用胺官能化 MOF 进行光开关 N-烷基化
催化 N-烷基化是合成仲胺和亚胺的常用方法,但选择性控制仍然是一个挑战,通常需要在恶劣的反应条件下使用专门的催化剂。在这里,我们提出了在温和条件下使用胺官能化金属有机框架(MIL-125-NH),用芳香族卤化物对胺进行光可切换的N-烷基化,以选择性合成仲胺和亚胺。 MIL-125-NH催化剂具有路易斯酸性位点,可催化溴化物与伯胺在暗处直接脱卤缩合生成仲胺。辐射后,MIL-125-NH 会还原分子氧,产生氧自由基,将溴化物转化为相应的醛,产生亚胺,与胺发生脱水偶联。该系统具有适当的酸度、快速的氧还原动力学以及对芳香族溴化物和生成水的优化吸附,可催化多种底物的转化,因此是一种有前景的应用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号