当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regulating the double-site Mn2-N6 electronic structure by manganese clusters for enhanced oxygen reduction
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.apcatb.2024.123939 Guangtao Luo , Enze Zhu , Chaoyang Shi , Yanrong Ren , Yan Lin , Xikun Yang , Mingli Xu
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.apcatb.2024.123939 Guangtao Luo , Enze Zhu , Chaoyang Shi , Yanrong Ren , Yan Lin , Xikun Yang , Mingli Xu
|
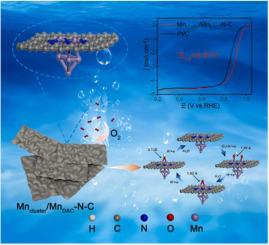
Effectively designing the coordination environment of metal atoms in single-atom catalysts to enhance the oxygen reduction reaction (ORR) performance is challenging. Herein, a strategy for regulating the electronic structure of double-site Mn-N by axial traction of Mn cluster is presented. The atomically dispersed homonuclear double-site Mn-N was synthesized. Experimental investigations and theoretical calculations revealed that the oxygen adsorption capacity of double-site Mn-N was stronger than that of Mn-N, which can improve the weak adsorption of oxygen at isolated Mn active sites. The introduction of Mn clusters broke the planar structure of Mn-N through axial traction, further altering the electron configuration around the active sites and effectively decreasing the adsorption strength of oxygen-containing intermediates and reaction energy barriers, leading to increased intrinsic activity, which greatly improved the ORR performance (E = 0.91 V). This work presented a novel way of regulating the electronic structure of homonuclear double single-atom sites by clusters.
中文翻译:

通过锰簇调控双位点Mn2-N6电子结构增强氧还原
有效设计单原子催化剂中金属原子的配位环境以提高氧还原反应(ORR)性能具有挑战性。在此,提出了一种通过Mn簇的轴向牵引来调节双位点Mn-N电子结构的策略。合成了原子分散的同核双位点Mn-N。实验研究和理论计算表明,双位点Mn-N的氧吸附能力强于Mn-N,可以改善孤立的Mn活性位点对氧的弱吸附。 Mn簇的引入通过轴向牵引打破了Mn-N的平面结构,进一步改变了活性位点周围的电子构型,有效降低了含氧中间体的吸附强度和反应能垒,导致本征活性增加,大大提高了Mn-N的性能。提高了 ORR 性能 (E = 0.91 V)。这项工作提出了一种通过簇调控同核双单原子位点电子结构的新方法。
更新日期:2024-03-11
中文翻译:

通过锰簇调控双位点Mn2-N6电子结构增强氧还原
有效设计单原子催化剂中金属原子的配位环境以提高氧还原反应(ORR)性能具有挑战性。在此,提出了一种通过Mn簇的轴向牵引来调节双位点Mn-N电子结构的策略。合成了原子分散的同核双位点Mn-N。实验研究和理论计算表明,双位点Mn-N的氧吸附能力强于Mn-N,可以改善孤立的Mn活性位点对氧的弱吸附。 Mn簇的引入通过轴向牵引打破了Mn-N的平面结构,进一步改变了活性位点周围的电子构型,有效降低了含氧中间体的吸附强度和反应能垒,导致本征活性增加,大大提高了Mn-N的性能。提高了 ORR 性能 (E = 0.91 V)。这项工作提出了一种通过簇调控同核双单原子位点电子结构的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号