当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Drug deconjugation-assisted peptide mapping by LC–MS/MS to identify conjugation sites and quantify site occupancy for antibody-drug conjugates
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.jpba.2024.116098 Tongdan Wang , Zi-Ao Huang , Moyin Zhou , Ruxin Wang , Yufei Li , Longyun Guo , Xiaolin Cao , Jincui Huang
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.jpba.2024.116098 Tongdan Wang , Zi-Ao Huang , Moyin Zhou , Ruxin Wang , Yufei Li , Longyun Guo , Xiaolin Cao , Jincui Huang
|
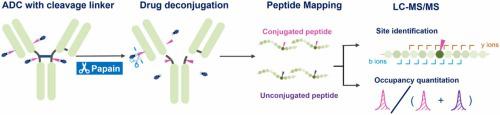
Antibody-drug conjugates (ADCs) are a heterogeneous mixture of conjugated species with varied drug loadings. Depending on conjugation sites, linkers and drugs can exhibit different stability as influenced by the solvent-accessibility and local charge, resulting in different ADC efficacy, pharmacokinetics, and toxicity. Conjugation site analysis is critical for ADC structural characterization to assure product quality and consistency. It enables early conjugation studies at site-specific levels, confirms the absence of unexpected products to support conjugation process development, and aids in ensuring lot-to-lot consistency for comparability studies. Peptide mapping using liquid chromatography-tandem mass spectrometry is the industry standard method for analyzing conjugation sites. However, some concerns remain for this approach as the large and hydrophobic drug moieties often result in poor MS/MS fragmentation quality and impede the identification of conjugation sites. Additionally, the ionization discrepancy between conjugated and unconjugated peptides can lead to a relatively large bias for site occupancy calculation. In this work, we present a simple drug deconjugation-assisted peptide mapping method to identify and quantify the drug conjugation for ADCs with protease-cleavable linkers. Papain-based drug deconjugation was used to remove the highly hydrophobic drug moiety, which significantly improved the quantitation accuracy of conjugation level and the fragmentation quality. Sample preparation conditions were optimized to avoid introducing artificial modifications, allowing the tracking of initial sample status and subsequent changes of quality attributes during process development and stability assessment. This method was applied to analyze thermally-stressed ADC samples to monitor changes of site-specific conjugation levels, DAR, succinimide hydrolysis of the linker, and various PTMs. We believe this is an effective and straightforward tool for conjugation site analysis while simultaneously monitoring multiple quality attributes for ADCs with protease-cleavable linkers.
中文翻译:

通过 LC-MS/MS 进行药物解偶联辅助肽图分析,以识别偶联位点并量化抗体-药物偶联物的位点占用率
抗体-药物偶联物 (ADC) 是具有不同载药量的偶联物的异质混合物。根据缀合位点,接头和药物可能会因溶剂可及性和局部电荷的影响而表现出不同的稳定性,从而导致不同的 ADC 功效、药代动力学和毒性。结合位点分析对于 ADC 结构表征至关重要,可确保产品质量和一致性。它能够在特定位点水平上进行早期缀合研究,确认不存在意外产物来支持缀合工艺开发,并有助于确保可比性研究的批次间一致性。使用液相色谱-串联质谱法进行肽图分析是分析缀合位点的行业标准方法。然而,这种方法仍然存在一些问题,因为大且疏水的药物部分通常会导致 MS/MS 碎片质量差并阻碍缀合位点的识别。此外,缀合肽和非缀合肽之间的电离差异可能导致位点占用计算出现相对较大的偏差。在这项工作中,我们提出了一种简单的药物解偶联辅助肽图分析方法,用于识别和量化具有蛋白酶可切割接头的 ADC 的药物偶联。采用基于木瓜蛋白酶的药物解离技术去除了高度疏水性的药物部分,显着提高了结合水平的定量精度和裂解质量。优化样品制备条件以避免引入人为修改,从而在工艺开发和稳定性评估过程中跟踪初始样品状态和质量属性的后续变化。该方法用于分析热应力 ADC 样品,以监测位点特异性缀合水平、DAR、接头的琥珀酰亚胺水解和各种 PTM 的变化。我们相信,这是一种有效且简单的工具,可用于缀合位点分析,同时监测具有蛋白酶可切割接头的 ADC 的多种质量属性。
更新日期:2024-03-07
中文翻译:

通过 LC-MS/MS 进行药物解偶联辅助肽图分析,以识别偶联位点并量化抗体-药物偶联物的位点占用率
抗体-药物偶联物 (ADC) 是具有不同载药量的偶联物的异质混合物。根据缀合位点,接头和药物可能会因溶剂可及性和局部电荷的影响而表现出不同的稳定性,从而导致不同的 ADC 功效、药代动力学和毒性。结合位点分析对于 ADC 结构表征至关重要,可确保产品质量和一致性。它能够在特定位点水平上进行早期缀合研究,确认不存在意外产物来支持缀合工艺开发,并有助于确保可比性研究的批次间一致性。使用液相色谱-串联质谱法进行肽图分析是分析缀合位点的行业标准方法。然而,这种方法仍然存在一些问题,因为大且疏水的药物部分通常会导致 MS/MS 碎片质量差并阻碍缀合位点的识别。此外,缀合肽和非缀合肽之间的电离差异可能导致位点占用计算出现相对较大的偏差。在这项工作中,我们提出了一种简单的药物解偶联辅助肽图分析方法,用于识别和量化具有蛋白酶可切割接头的 ADC 的药物偶联。采用基于木瓜蛋白酶的药物解离技术去除了高度疏水性的药物部分,显着提高了结合水平的定量精度和裂解质量。优化样品制备条件以避免引入人为修改,从而在工艺开发和稳定性评估过程中跟踪初始样品状态和质量属性的后续变化。该方法用于分析热应力 ADC 样品,以监测位点特异性缀合水平、DAR、接头的琥珀酰亚胺水解和各种 PTM 的变化。我们相信,这是一种有效且简单的工具,可用于缀合位点分析,同时监测具有蛋白酶可切割接头的 ADC 的多种质量属性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号