当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hybrid molecules synergistically mitigate ferroptosis and amyloid-associated toxicities in Alzheimer's disease
Redox Biology ( IF 11.4 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.redox.2024.103119 Dikshaa Padhi , Prayasee Baruah , Madhu Ramesh , Hariharan Moorthy , Thimmaiah Govindaraju
Redox Biology ( IF 11.4 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.redox.2024.103119 Dikshaa Padhi , Prayasee Baruah , Madhu Ramesh , Hariharan Moorthy , Thimmaiah Govindaraju
|
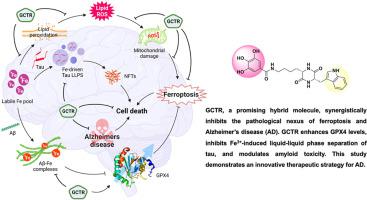
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by the build-up of extracellular amyloid β (Aβ) plaques and intracellular neurofibrillary tangles (NFTs). Ferroptosis, an iron (Fe)-dependent form of cell death plays a significant role in the multifaceted AD pathogenesis through generation of reactive oxygen species (ROS), mitochondrial damage, lipid peroxidation, and reduction in glutathione peroxidase 4 (GPX4) enzyme activity and levels. Aberrant liquid-liquid phase separation (LLPS) of tau drives the growth and maturation of NFTs contributing to AD pathogenesis. In this study, we strategically combined the structural and functional properties of gallic acid (GA) and cyclic dipeptides (CDPs) to synthesize hybrid molecules that effectively target both ferroptosis and amyloid toxicity in AD. This innovative approach marks a paradigm shift from conventional therapeutic strategies. This is the first report of a synthetic small molecule () that effectively combats ferroptosis, simultaneously restoring enzymatic activity and enhancing cellular levels of its master regulator, GPX4. Further, disrupts Fe-induced LLPS of tau, and aids in attenuation of abnormal tau fibrillization. The synergistic action of in combating both ferroptosis and amyloid toxicity, bolstered by GPX4 enhancement and modulation of Fe-induced tau LLPS, holds promise for the development of small molecule-based novel therapeutics for AD.
中文翻译:

混合分子协同减轻阿尔茨海默病中的铁死亡和淀粉样蛋白相关毒性
阿尔茨海默病 (AD) 是一种神经退行性疾病,其特征是细胞外 β 淀粉样蛋白 (Aβ) 斑块和细胞内神经原纤维缠结 (NFT) 的形成。铁死亡是一种铁 (Fe) 依赖性细胞死亡形式,通过产生活性氧 (ROS)、线粒体损伤、脂质过氧化和谷胱甘肽过氧化物酶 4 (GPX4) 酶活性降低,在多方面 AD 发病机制中发挥着重要作用。水平。 tau 蛋白的异常液-液相分离 (LLPS) 会促进 NFT 的生长和成熟,从而导致 AD 发病。在这项研究中,我们策略性地结合了没食子酸(GA)和环状二肽(CDP)的结构和功能特性,合成了能够有效靶向 AD 中铁死亡和淀粉样蛋白毒性的混合分子。这种创新方法标志着传统治疗策略的范式转变。这是第一份关于有效对抗铁死亡的合成小分子 () 的报告,同时恢复酶活性并增强其主调节因子 GPX4 的细胞水平。此外,还可破坏 Fe 诱导的 tau 蛋白 LLPS,并有助于减弱异常 tau 蛋白原纤维化。在 GPX4 增强和 Fe 诱导的 tau LLPS 调节的支持下,对抗铁死亡和淀粉样蛋白毒性的协同作用为开发基于小分子的新型 AD 疗法带来了希望。
更新日期:2024-03-11
中文翻译:

混合分子协同减轻阿尔茨海默病中的铁死亡和淀粉样蛋白相关毒性
阿尔茨海默病 (AD) 是一种神经退行性疾病,其特征是细胞外 β 淀粉样蛋白 (Aβ) 斑块和细胞内神经原纤维缠结 (NFT) 的形成。铁死亡是一种铁 (Fe) 依赖性细胞死亡形式,通过产生活性氧 (ROS)、线粒体损伤、脂质过氧化和谷胱甘肽过氧化物酶 4 (GPX4) 酶活性降低,在多方面 AD 发病机制中发挥着重要作用。水平。 tau 蛋白的异常液-液相分离 (LLPS) 会促进 NFT 的生长和成熟,从而导致 AD 发病。在这项研究中,我们策略性地结合了没食子酸(GA)和环状二肽(CDP)的结构和功能特性,合成了能够有效靶向 AD 中铁死亡和淀粉样蛋白毒性的混合分子。这种创新方法标志着传统治疗策略的范式转变。这是第一份关于有效对抗铁死亡的合成小分子 () 的报告,同时恢复酶活性并增强其主调节因子 GPX4 的细胞水平。此外,还可破坏 Fe 诱导的 tau 蛋白 LLPS,并有助于减弱异常 tau 蛋白原纤维化。在 GPX4 增强和 Fe 诱导的 tau LLPS 调节的支持下,对抗铁死亡和淀粉样蛋白毒性的协同作用为开发基于小分子的新型 AD 疗法带来了希望。



























 京公网安备 11010802027423号
京公网安备 11010802027423号