当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rational Design of TDP-43 Derived α-Helical Peptide Inhibitors: An In Silico Strategy to Prevent TDP-43 Aggregation in Neurodegenerative Disorders
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2024-03-11 , DOI: 10.1021/acschemneuro.3c00659 Muthu Raj Salaikumaran 1 , Pallavi P. Gopal 1, 2
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2024-03-11 , DOI: 10.1021/acschemneuro.3c00659 Muthu Raj Salaikumaran 1 , Pallavi P. Gopal 1, 2
Affiliation
|
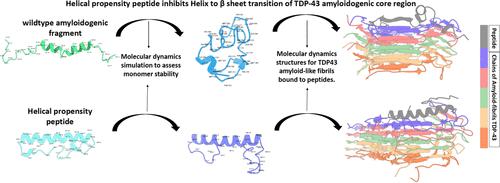
TDP-43, an essential RNA/DNA-binding protein, is central to the pathology of neurodegenerative diseases, such as amyotrophic lateral sclerosis and frontotemporal dementia. Pathological mislocalization and aggregation of TDP-43 disrupt RNA splicing, mRNA stability, and mRNA transport, thereby impairing neuronal function and survival. The formation of amyloid-like TDP-43 filaments is largely facilitated by the destabilization of an α-helical segment within the disordered C-terminal region. In this study, we hypothesized that preventing the destabilization of the α-helical domain could potentially halt the growth of these pathological filaments. To explore this, we utilized a range of in silico techniques to design and evaluate peptide-based therapeutics that bind to pathological TDP-43 amyloid-like filament crystal structures and resist β sheet conversion. Our computational approaches, including biophysical and secondary structure property prediction, molecular docking, 3D structure prediction, and molecular dynamics simulations, were used to assess the structure, stability, and binding affinity of these peptides in relation to pathological TDP-43 filaments. The results of our in silico analyses identified a selection of promising peptides which displayed a stable α-helical structure, exhibited an increased number of intramolecular hydrogen bonds within the helical domain, and demonstrated high binding affinities for pathological TDP-43 amyloid-like filaments. Molecular dynamics simulations provided further support for the structural and thermodynamic stability of these peptides, as they exhibited lower root-mean-square deviation and more favorable free energy landscapes over 300 ns. These findings establish α-helical propensity peptides as potential lead molecules for the development of novel therapeutics against TDP-43 aggregation. This structure-based computational approach for the rational design of peptide inhibitors opens a new direction in the search for effective interventions for ALS, FTD, and other related neurodegenerative diseases. The peptides identified as the most promising candidates in this study are currently subject to further testing and validation through both in vitro and in vivo experiments.
中文翻译:

TDP-43 衍生的 α-螺旋肽抑制剂的合理设计:防止神经退行性疾病中 TDP-43 聚集的计算机策略
TDP-43 是一种必需的 RNA/DNA 结合蛋白,是神经退行性疾病(例如肌萎缩侧索硬化症和额颞叶痴呆)病理学的核心。TDP-43 的病理性错误定位和聚集会破坏 RNA 剪接、mRNA 稳定性和 mRNA 运输,从而损害神经元功能和存活。淀粉样蛋白样 TDP-43 丝的形成很大程度上是通过无序 C 末端区域内 α 螺旋片段的不稳定来促进的。在这项研究中,我们假设防止 α-螺旋结构域的不稳定可能会阻止这些病理丝的生长。为了探索这一点,我们利用一系列计算机技术来设计和评估基于肽的疗法,这些疗法与病理性 TDP-43 淀粉样蛋白样丝状晶体结构结合并抵抗 β 片层转化。我们的计算方法,包括生物物理和二级结构特性预测、分子对接、3D结构预测和分子动力学模拟,用于评估这些肽与病理性TDP-43丝的结构、稳定性和结合亲和力。我们的计算机分析结果确定了一系列有前景的肽,这些肽表现出稳定的 α-螺旋结构,螺旋结构域内的分子内氢键数量增加,并表现出对病理性 TDP-43 淀粉样蛋白丝的高结合亲和力。分子动力学模拟为这些肽的结构和热力学稳定性提供了进一步的支持,因为它们在 300 ns 内表现出较低的均方根偏差和更有利的自由能景观。这些发现确立了 α-螺旋倾向肽作为开发针对 TDP-43 聚集的新型疗法的潜在先导分子。这种基于结构的计算方法用于合理设计肽抑制剂,为寻找 ALS、FTD 和其他相关神经退行性疾病的有效干预措施开辟了新方向。本研究中被确定为最有希望的候选肽目前正在通过体外和体内实验进行进一步的测试和验证。
更新日期:2024-03-11
中文翻译:

TDP-43 衍生的 α-螺旋肽抑制剂的合理设计:防止神经退行性疾病中 TDP-43 聚集的计算机策略
TDP-43 是一种必需的 RNA/DNA 结合蛋白,是神经退行性疾病(例如肌萎缩侧索硬化症和额颞叶痴呆)病理学的核心。TDP-43 的病理性错误定位和聚集会破坏 RNA 剪接、mRNA 稳定性和 mRNA 运输,从而损害神经元功能和存活。淀粉样蛋白样 TDP-43 丝的形成很大程度上是通过无序 C 末端区域内 α 螺旋片段的不稳定来促进的。在这项研究中,我们假设防止 α-螺旋结构域的不稳定可能会阻止这些病理丝的生长。为了探索这一点,我们利用一系列计算机技术来设计和评估基于肽的疗法,这些疗法与病理性 TDP-43 淀粉样蛋白样丝状晶体结构结合并抵抗 β 片层转化。我们的计算方法,包括生物物理和二级结构特性预测、分子对接、3D结构预测和分子动力学模拟,用于评估这些肽与病理性TDP-43丝的结构、稳定性和结合亲和力。我们的计算机分析结果确定了一系列有前景的肽,这些肽表现出稳定的 α-螺旋结构,螺旋结构域内的分子内氢键数量增加,并表现出对病理性 TDP-43 淀粉样蛋白丝的高结合亲和力。分子动力学模拟为这些肽的结构和热力学稳定性提供了进一步的支持,因为它们在 300 ns 内表现出较低的均方根偏差和更有利的自由能景观。这些发现确立了 α-螺旋倾向肽作为开发针对 TDP-43 聚集的新型疗法的潜在先导分子。这种基于结构的计算方法用于合理设计肽抑制剂,为寻找 ALS、FTD 和其他相关神经退行性疾病的有效干预措施开辟了新方向。本研究中被确定为最有希望的候选肽目前正在通过体外和体内实验进行进一步的测试和验证。



























 京公网安备 11010802027423号
京公网安备 11010802027423号