当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium for Ethyl Acetate + Acetonitrile with Acetate-Based Ionic Liquids as Entrainers at 101.3 kPa and Analysis of the Separation Mechanism
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-08 , DOI: 10.1021/acs.jced.3c00664 Kun Yue 1 , Jiani Lu 1 , Peiyu Chen 1 , Shaonan Gu 1 , Guowei Zhou 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-08 , DOI: 10.1021/acs.jced.3c00664 Kun Yue 1 , Jiani Lu 1 , Peiyu Chen 1 , Shaonan Gu 1 , Guowei Zhou 1
Affiliation
|
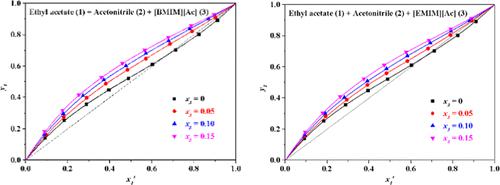
Isobaric vapor–liquid equilibrium (VLE) data for the ternary system of ethyl acetate + acetonitrile + acetate-based ionic liquids (ILs) were obtained with a modified Othmer still at 101.3 kPa, and the used ILs were 1-butyl-3-methylimidazolium acetate ([BMIM][Ac]) and 1-ethyl-3-methylimidazolium acetate ([EMIM][Ac]). The VLE data were correlated with the nonrandom two-liquid model and fitted well, indicating that the minimum mole fractions of [BMIM][Ac] and [EMIM][Ac] to eliminate azeotrope were 0.028 and 0.067, respectively. Both [BMIM][Ac] and [EMIM][Ac] led to a salting-out effect on ethyl acetate, and a higher concentration of ILs signified a stronger salt effect. In addition, interactions between ILs and solvents were estimated, and the polarity was analyzed with the σ-profiles, revealing the effect of ILs and the separation mechanism of the ternary system. The results indicated that both [BMIM][Ac] and [EMIM][Ac] could be qualified as solvents to separate ethyl acetate and acetonitrile. Based on the experimental data measured on the equilibrium scale and the separation mechanism calculated at the molecular scale, this work provides a useful strategy for studying the application of IL in extractive distillation in the future.
中文翻译:

以醋酸盐离子液体为夹带剂的醋酸乙酯+乙腈在101.3 kPa下的气液平衡及分离机理分析
乙酸乙酯+乙腈+乙酸基离子液体(IL)三元体系的等压气液平衡(VLE)数据是用改进的Othmer蒸馏器在101.3 kPa下获得的,所用离子液体为1-丁基-3-甲基咪唑鎓乙酸盐 ([BMIM][Ac]) 和 1-乙基-3-甲基咪唑鎓乙酸盐 ([EMIM][Ac])。 VLE数据与非随机双液模型相关且拟合良好,表明消除共沸物的[BMIM][Ac]和[EMIM][Ac]的最小摩尔分数分别为0.028和0.067。 [BMIM][Ac]和[EMIM][Ac]均对乙酸乙酯产生盐析作用,ILs浓度越高,盐析作用越强。此外,还估计了离子液体和溶剂之间的相互作用,并用σ分布分析了极性,揭示了离子液体的作用和三元体系的分离机制。结果表明[BMIM][Ac]和[EMIM][Ac]均可作为分离乙酸乙酯和乙腈的溶剂。基于平衡尺度上测量的实验数据和分子尺度上计算的分离机理,这项工作为研究离子液体在萃取精馏中的应用提供了有用的策略。
更新日期:2024-03-08
中文翻译:

以醋酸盐离子液体为夹带剂的醋酸乙酯+乙腈在101.3 kPa下的气液平衡及分离机理分析
乙酸乙酯+乙腈+乙酸基离子液体(IL)三元体系的等压气液平衡(VLE)数据是用改进的Othmer蒸馏器在101.3 kPa下获得的,所用离子液体为1-丁基-3-甲基咪唑鎓乙酸盐 ([BMIM][Ac]) 和 1-乙基-3-甲基咪唑鎓乙酸盐 ([EMIM][Ac])。 VLE数据与非随机双液模型相关且拟合良好,表明消除共沸物的[BMIM][Ac]和[EMIM][Ac]的最小摩尔分数分别为0.028和0.067。 [BMIM][Ac]和[EMIM][Ac]均对乙酸乙酯产生盐析作用,ILs浓度越高,盐析作用越强。此外,还估计了离子液体和溶剂之间的相互作用,并用σ分布分析了极性,揭示了离子液体的作用和三元体系的分离机制。结果表明[BMIM][Ac]和[EMIM][Ac]均可作为分离乙酸乙酯和乙腈的溶剂。基于平衡尺度上测量的实验数据和分子尺度上计算的分离机理,这项工作为研究离子液体在萃取精馏中的应用提供了有用的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号