Chem ( IF 23.5 ) Pub Date : 2024-02-29 , DOI: 10.1016/j.chempr.2024.02.006 Jiandong Liu , Wen-Bin Cao , Shu-Li You
|
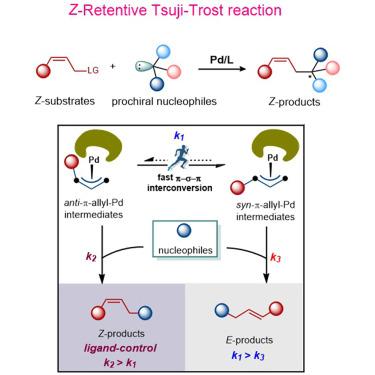
The palladium-catalyzed allylic substitution (Tsuji-Trost) reaction is widely applied in organic synthesis, especially for the synthesis of stereochemically well-defined olefins. However, the synthesis of Z-olefins via the Tsuji-Trost reaction has been challenging due to the thermodynamic instability of the corresponding anti-π-allyl-palladium intermediate. Here, we report a ligand-enabled palladium-catalyzed Z-retentive allylic substitution reaction that retains Z-olefin geometries. Palladium catalysts derived from sterically bulky phosphoramidite ligands well differentiate the reaction rates between the nucleophilic attack step and the π-σ-π isomerization process. The Z-retentive allylic substitution results from the nucleophilic attack occurring much faster than the isomerization process. The isomerization of anti-π-allyl-palladium intermediate into its syn-counterpart has been observed at a low temperature. These results provide a prospective approach for the preparation of chiral Z-olefin compounds.
中文翻译:

配体支持的 Z 保留 Tsuji-Trost 反应
钯催化的烯丙基取代(Tsuji-Trost)反应广泛应用于有机合成,特别是立体化学结构明确的烯烃的合成。然而,由于相应的反-π-烯丙基-钯中间体的热力学不稳定性,通过Tsuji-Trost反应合成Z-烯烃一直具有挑战性。在这里,我们报道了一种配体钯催化的Z保留烯丙基取代反应,该反应保留了Z烯烃的几何形状。由空间大的亚磷酰胺配体衍生的钯催化剂可以很好地区分亲核攻击步骤和 π-σ-π 异构化过程之间的反应速率。 Z保留烯丙基取代是由比异构化过程发生得快得多的亲核攻击引起的。已在低温下观察到反-π-烯丙基-钯中间体异构化为其顺式对应物。这些结果为手性Z-烯烃化合物的制备提供了一种有前景的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号