当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of Excipient Extraction and Buffer Exchange on Recombinant Monoclonal Antibody Stability
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.molpharmaceut.3c01157 Deepika Sarin 1 , Kunal Krishna 2 , M. Reza Nejadnik 3 , Raj Suryanarayanan 4 , Anurag S. Rathore 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.molpharmaceut.3c01157 Deepika Sarin 1 , Kunal Krishna 2 , M. Reza Nejadnik 3 , Raj Suryanarayanan 4 , Anurag S. Rathore 1
Affiliation
|
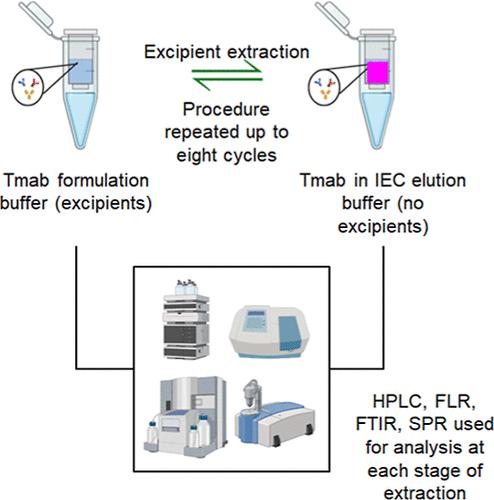
The foundation of a biosimilar manufacturer’s regulatory filing is the demonstration of analytical and functional similarity between the biosimilar product and the pertinent originator product. The excipients in the formulation may interfere with characterization using typical analytical and functional techniques during this biosimilarity exercise. Consequently, the producers of biosimilar products resort to buffer exchange to isolate the biotherapeutic protein from the drug product formulation. However, the impact that this isolation has on the product stability is not completely known. This study aims to elucidate the extent to which mAb isolation via ultrafiltration-diafiltration-based buffer exchange impacts mAb stability. It has been demonstrated that repeated extraction cycles do result in significant changes in higher-order structure (red-shift of 5.0 nm in fluorescence maxima of buffer exchanged samples) of the mAb and also an increase in formation of basic variants from 19.1 to 26.7% and from 32.3 to 36.9% in extracted innovator and biosimilar Tmab samples, respectively. It was also observed that under certain conditions of tertiary structure disruptions, Tmab could be restabilized depending on formulation composition. Thus, mAb isolation through extraction with buffer exchange impacts the product stability. Based on the observations reported in this paper, we recommend that biosimilar manufacturers take into consideration these effects of excipients on protein stability when performing biosimilarity assessments.
中文翻译:

赋形剂提取和缓冲液更换对重组单克隆抗体稳定性的影响
生物仿制药制造商监管备案的基础是证明生物仿制药产品与相关原研产品之间的分析和功能相似性。制剂中的赋形剂可能会干扰生物相似性试验期间使用典型分析和功能技术进行的表征。因此,生物仿制药产品的生产商采用缓冲液交换来从药品配方中分离生物治疗蛋白质。然而,这种隔离对产品稳定性的影响尚不完全清楚。本研究旨在阐明通过基于超滤-渗滤的缓冲液交换进行的 mAb 分离对 mAb 稳定性的影响程度。已经证明,重复的提取循环确实会导致 mAb 的高阶结构发生显着变化(缓冲液交换样品的荧光最大值红移 5.0 nm),并且基本变体的形成从 19.1% 增加到 26.7%提取的创新药和生物仿制药 Tmab 样品中的含量分别从 32.3% 提高到 36.9%。还观察到,在三级结构破坏的某些条件下,Tmab 可以根据制剂组成重新稳定。因此,通过缓冲液交换提取来分离 mAb 会影响产品的稳定性。根据本文报告的观察结果,我们建议生物仿制药制造商在进行生物相似性评估时考虑辅料对蛋白质稳定性的影响。
更新日期:2024-02-29
中文翻译:

赋形剂提取和缓冲液更换对重组单克隆抗体稳定性的影响
生物仿制药制造商监管备案的基础是证明生物仿制药产品与相关原研产品之间的分析和功能相似性。制剂中的赋形剂可能会干扰生物相似性试验期间使用典型分析和功能技术进行的表征。因此,生物仿制药产品的生产商采用缓冲液交换来从药品配方中分离生物治疗蛋白质。然而,这种隔离对产品稳定性的影响尚不完全清楚。本研究旨在阐明通过基于超滤-渗滤的缓冲液交换进行的 mAb 分离对 mAb 稳定性的影响程度。已经证明,重复的提取循环确实会导致 mAb 的高阶结构发生显着变化(缓冲液交换样品的荧光最大值红移 5.0 nm),并且基本变体的形成从 19.1% 增加到 26.7%提取的创新药和生物仿制药 Tmab 样品中的含量分别从 32.3% 提高到 36.9%。还观察到,在三级结构破坏的某些条件下,Tmab 可以根据制剂组成重新稳定。因此,通过缓冲液交换提取来分离 mAb 会影响产品的稳定性。根据本文报告的观察结果,我们建议生物仿制药制造商在进行生物相似性评估时考虑辅料对蛋白质稳定性的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号