当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneous determination of iguratimod and its metabolite in rat plasma using a UPLC-MS/MS method: Application for drug-drug interaction
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2024-03-02 , DOI: 10.1016/j.jpba.2024.116079 Lu Shi , Jinyu Hu , Hualu Wu , Yuxin Shen , Xiaohai Chen , Qinghua Weng , Ren-ai Xu , Congrong Tang
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2024-03-02 , DOI: 10.1016/j.jpba.2024.116079 Lu Shi , Jinyu Hu , Hualu Wu , Yuxin Shen , Xiaohai Chen , Qinghua Weng , Ren-ai Xu , Congrong Tang
|
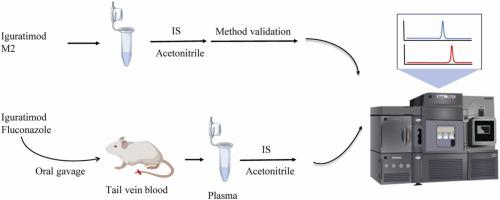
This aim of the work was to establish an acceptable sensitive assay based on ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) for quantitatively analyzing the plasma concentrations of iguratimod (IGR) and its metabolite M2 in rats, and to further investigate the effect of fluconazole on the pharmacokinetics of IGR and M2. The mobile phase consisted of acetonitrile and water with 0.1% formic acid, was used to separate IGR, M2 and internal standard (IS) fedratinib on a UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm) with the flow rate of 0.4 mL/min. Positive ion mode and multiple reaction monitoring (MRM) were used to construct the quantitative analysis. The calibration standard of IGR and M2 covered 2–10000 and 1–1000 ng/mL respectively, with the lower limit of quantification (LLOQ) as 2 ng/mL and 1 ng/mL respectively. In addition, selectivity, recovery, accuracy, precision, matrix effect and stability of the method validation program were well accepted in this work. Subsequently, this approach was used to assess the effect of fluconazole on the pharmacokinetics of IGR and M2 in rats. In the presence of 20 mg/kg fluconazole (experimental group), we found the main pharmacokinetic parameters were significantly altered when compared with 2.5 mg/kg IGR alone (control group). Among them, AUC and C of IGR in the experimental group was 1.43 and 1.08 times higher than that of the control group, respectively. Moreover, we also found that the other main pharmacokinetic parameters of M2 had no significant changes, except t and T. In conclusion, fluconazole significantly altered the main pharmacokinetics of IGR and M2 in rats. It implys that we should pay more attention to the adverse reaction of IGR when the concomitant use of fluconazole and IGR occur in the future clinical practice.
中文翻译:

使用 UPLC-MS/MS 方法同时测定大鼠血浆中艾拉莫德及其代谢物:在药物相互作用中的应用
本工作的目的是建立一种基于超高效液相色谱串联质谱 (UPLC-MS/MS) 的可接受的灵敏测定方法,用于定量分析大鼠体内艾拉莫德 (IGR) 及其代谢物 M2 的血浆浓度,并进一步研究氟康唑对 IGR 和 M2 药代动力学的影响。流动相为乙腈和水(含0.1%甲酸),在UPLC BEH C18柱(2.1 mm×50 mm,1.7 μm)上分离IGR、M2和内标(IS)fedratinib,流速为0.4毫升/分钟。使用正离子模式和多反应监测(MRM)构建定量分析。 IGR和M2的校准标准品分别覆盖2-10000和1-1000 ng/mL,定量下限(LLOQ)分别为2 ng/mL和1 ng/mL。此外,方法验证程序的选择性、回收率、准确度、精密度、基质效应和稳定性在本工作中得到了很好的认可。随后,该方法用于评估氟康唑对大鼠 IGR 和 M2 药代动力学的影响。在存在 20 mg/kg 氟康唑(实验组)的情况下,我们发现与单独使用 2.5 mg/kg IGR(对照组)相比,主要药代动力学参数发生了显着改变。其中,实验组IGR的AUC和Cmax分别是对照组的1.43和1.08倍。此外,我们还发现除t和T外,M2的其他主要药代动力学参数没有显着变化。综上所述,氟康唑显着改变了IGR和M2在大鼠体内的主要药代动力学。提示今后临床中氟康唑与IGR合用时应高度重视IGR的不良反应。
更新日期:2024-03-02
中文翻译:

使用 UPLC-MS/MS 方法同时测定大鼠血浆中艾拉莫德及其代谢物:在药物相互作用中的应用
本工作的目的是建立一种基于超高效液相色谱串联质谱 (UPLC-MS/MS) 的可接受的灵敏测定方法,用于定量分析大鼠体内艾拉莫德 (IGR) 及其代谢物 M2 的血浆浓度,并进一步研究氟康唑对 IGR 和 M2 药代动力学的影响。流动相为乙腈和水(含0.1%甲酸),在UPLC BEH C18柱(2.1 mm×50 mm,1.7 μm)上分离IGR、M2和内标(IS)fedratinib,流速为0.4毫升/分钟。使用正离子模式和多反应监测(MRM)构建定量分析。 IGR和M2的校准标准品分别覆盖2-10000和1-1000 ng/mL,定量下限(LLOQ)分别为2 ng/mL和1 ng/mL。此外,方法验证程序的选择性、回收率、准确度、精密度、基质效应和稳定性在本工作中得到了很好的认可。随后,该方法用于评估氟康唑对大鼠 IGR 和 M2 药代动力学的影响。在存在 20 mg/kg 氟康唑(实验组)的情况下,我们发现与单独使用 2.5 mg/kg IGR(对照组)相比,主要药代动力学参数发生了显着改变。其中,实验组IGR的AUC和Cmax分别是对照组的1.43和1.08倍。此外,我们还发现除t和T外,M2的其他主要药代动力学参数没有显着变化。综上所述,氟康唑显着改变了IGR和M2在大鼠体内的主要药代动力学。提示今后临床中氟康唑与IGR合用时应高度重视IGR的不良反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号