当前位置:
X-MOL 学术
›
Int. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy of ramucirumab combination chemotherapy as second-line treatment in patients with advanced adenocarcinoma of the stomach or gastroesophageal junction after exposure to checkpoint inhibitors and chemotherapy as first-line therapy
International Journal of Cancer ( IF 6.4 ) Pub Date : 2024-03-06 , DOI: 10.1002/ijc.34894 Michael Masetti 1 , Salah‐Eddin Al‐Batran 2 , Thorsten O. Goetze 2 , Peter Thuss‐Patience 3 , Jorge Riera Knorrenschild 4 , Eray Goekkurt 5 , Gunnar Folprecht 6 , Thomas Jens Ettrich 7 , Udo Lindig 8 , Kim Barbara Luley 9 , Daniel Pink 10 , Tobias Dechow 11 , Disorn Sookthai 12 , Sabine Junge 12 , Maria Loose 12 , Claudia Pauligk 12 , Sylvie Lorenzen 1
International Journal of Cancer ( IF 6.4 ) Pub Date : 2024-03-06 , DOI: 10.1002/ijc.34894 Michael Masetti 1 , Salah‐Eddin Al‐Batran 2 , Thorsten O. Goetze 2 , Peter Thuss‐Patience 3 , Jorge Riera Knorrenschild 4 , Eray Goekkurt 5 , Gunnar Folprecht 6 , Thomas Jens Ettrich 7 , Udo Lindig 8 , Kim Barbara Luley 9 , Daniel Pink 10 , Tobias Dechow 11 , Disorn Sookthai 12 , Sabine Junge 12 , Maria Loose 12 , Claudia Pauligk 12 , Sylvie Lorenzen 1
Affiliation
|
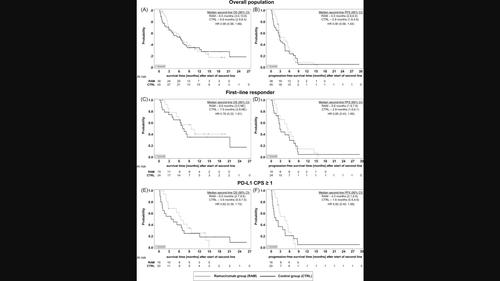
FOLFOX plus nivolumab represents a standard of care for first-line therapy of advanced gastroesophageal cancer (aGEC) with positive PD-L1 expression. The efficacy of second-line VEGFR-2 inhibition with ramucirumab (RAM) plus chemotherapy after progression to immunochemotherapy remains unclear. Medical records of patients with aGEC enrolled in the randomized phase II AIO-STO-0417 trial after treatment failure to first-line FOLFOX plus nivolumab and ipilimumab were retrospectively analyzed. Patients were divided into two groups based on second-line therapy: RAM plus chemotherapy (RAM group) or treatment without RAM (control group). Eighty three patients were included. In the overall population, progression-free survival (PFS) in the RAM group was superior to the control (4.5 vs 2.9 months). Responders (CR/PR) to first-line immunochemotherapy receiving RAM containing second-line therapy had prolonged OS from start of first-line therapy (28.9 vs 16.5 months), as well as second-line OS (9.6 vs 7.5 months), PFS (5.6 vs 2.9 months) and DCR (53% vs 29%) compared to the control. PD-L1 CPS ≥1 was 42% and 44% for the RAM and the control, respectively. Patients with CPS ≥1 in the RAM group showed better tumor control (ORR 25% vs 10%) and improved survival (total OS 11.5 vs 8.0 months; second-line OS 6.5 vs 3.9 months; PFS 4.5 vs 1.6 months) compared to the control. Prior exposure to first-line FOLFOX plus dual checkpoint inhibition followed by RAM plus chemotherapy shows favorable response and survival rates especially in patients with initial response and positive PD-L1 expression and has the potential to advance the treatment paradigm in aGEC.
中文翻译:

雷莫芦单抗联合化疗作为二线治疗对接受检查点抑制剂和化疗作为一线治疗的晚期胃或胃食管连接部腺癌患者的疗效
FOLFOX 加纳武单抗代表了 PD-L1 表达阳性的晚期胃食管癌 (aGEC) 的一线治疗标准。进展为免疫化疗后,使用雷莫芦单抗 (RAM) 联合化疗进行二线 VEGFR-2 抑制的疗效仍不清楚。对一线 FOLFOX 加纳武单抗和易普利姆玛治疗失败后参加随机 II 期 AIO-STO-0417 试验的 aGEC 患者的医疗记录进行了回顾性分析。根据二线治疗将患者分为两组:RAM加化疗(RAM组)或不使用RAM治疗(对照组)。其中包括八十三名患者。在总体人群中,RAM 组的无进展生存期 (PFS) 优于对照组(4.5 个月 vs 2.9 个月)。对接受包含二线治疗的 RAM 的一线免疫化疗有反应的患者 (CR/PR) 自一线治疗开始后 OS 延长(28.9 个月 vs 16.5 个月),二线 OS 延长(9.6 个月 vs 7.5 个月),PFS 也延长与对照组相比,(5.6 个月 vs 2.9 个月)和 DCR(53% vs 29%)。 RAM 和对照组的 PD-L1 CPS ≥1 分别为 42% 和 44%。与对照组相比,RAM 组 CPS ≥1 的患者表现出更好的肿瘤控制(ORR 25% vs 10%)和改善的生存率(总 OS 11.5 vs 8.0 个月;二线 OS 6.5 vs 3.9 个月;PFS 4.5 vs 1.6 个月)控制。先前接触一线 FOLFOX 加双重检查点抑制,随后 RAM 加化疗显示出良好的反应和生存率,特别是在具有初始反应和阳性 PD-L1 表达的患者中,并且有潜力推进 aGEC 的治疗模式。
更新日期:2024-03-06
中文翻译:

雷莫芦单抗联合化疗作为二线治疗对接受检查点抑制剂和化疗作为一线治疗的晚期胃或胃食管连接部腺癌患者的疗效
FOLFOX 加纳武单抗代表了 PD-L1 表达阳性的晚期胃食管癌 (aGEC) 的一线治疗标准。进展为免疫化疗后,使用雷莫芦单抗 (RAM) 联合化疗进行二线 VEGFR-2 抑制的疗效仍不清楚。对一线 FOLFOX 加纳武单抗和易普利姆玛治疗失败后参加随机 II 期 AIO-STO-0417 试验的 aGEC 患者的医疗记录进行了回顾性分析。根据二线治疗将患者分为两组:RAM加化疗(RAM组)或不使用RAM治疗(对照组)。其中包括八十三名患者。在总体人群中,RAM 组的无进展生存期 (PFS) 优于对照组(4.5 个月 vs 2.9 个月)。对接受包含二线治疗的 RAM 的一线免疫化疗有反应的患者 (CR/PR) 自一线治疗开始后 OS 延长(28.9 个月 vs 16.5 个月),二线 OS 延长(9.6 个月 vs 7.5 个月),PFS 也延长与对照组相比,(5.6 个月 vs 2.9 个月)和 DCR(53% vs 29%)。 RAM 和对照组的 PD-L1 CPS ≥1 分别为 42% 和 44%。与对照组相比,RAM 组 CPS ≥1 的患者表现出更好的肿瘤控制(ORR 25% vs 10%)和改善的生存率(总 OS 11.5 vs 8.0 个月;二线 OS 6.5 vs 3.9 个月;PFS 4.5 vs 1.6 个月)控制。先前接触一线 FOLFOX 加双重检查点抑制,随后 RAM 加化疗显示出良好的反应和生存率,特别是在具有初始反应和阳性 PD-L1 表达的患者中,并且有潜力推进 aGEC 的治疗模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号