当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Akedanones A–C, In Vitro and In Vivo Antiplasmodial 2,3-Dihydronaphthoquinones Produced by Streptomyces sp. K20-0187
Journal of Natural Products ( IF 5.1 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.jnatprod.3c01285 So-ichiro Kimura 1 , Yoshihiro Watanabe 1, 2 , Yuta Kikuchi 1 , Shiori Shibasaki 2 , Hayama Tsutsumi 1, 2 , Yuki Inahashi 1, 2 , Rei Hokari 1, 2 , Aki Ishiyama 1, 2 , Masato Iwatsuki 1, 2
Journal of Natural Products ( IF 5.1 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.jnatprod.3c01285 So-ichiro Kimura 1 , Yoshihiro Watanabe 1, 2 , Yuta Kikuchi 1 , Shiori Shibasaki 2 , Hayama Tsutsumi 1, 2 , Yuki Inahashi 1, 2 , Rei Hokari 1, 2 , Aki Ishiyama 1, 2 , Masato Iwatsuki 1, 2
Affiliation
|
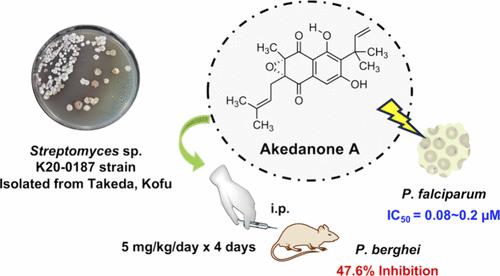
Three new antiplasmodial compounds, named akedanones A (1), B (2), and C (3), were discovered from the cultured material of Streptomyces sp. K20-0187 isolated from a soil sample collected at Takeda, Kofu, Yamanashi prefecture in Japan. The structures of compounds 1–3 were elucidated as new 2,3-dihydronaphthoquinones having prenyl and reverse prenyl groups by mass spectrometry and nuclear magnetic resonance analyses. Compound 1 and the known furanonaphthoquinone I (4) showed potent in vitro antiplasmodial activity against chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum strains, with half-maximal inhibitory concentration values ranging from 0.06 to 0.3 μM. Compounds 1 and 4 also displayed potent in vivo antiplasmodial activity against drug-sensitive rodent malaria Plasmodium berghei N strain, with inhibition rates of 47.6 and 43.1%, respectively, on intraperitoneal administration at a dose of 5 mg kg–1 day–1 for 4 days.
中文翻译:

Akedanones A–C,体外和体内 抗疟原虫 2,3-二氢萘醌 由链霉菌产生。 K20-0187
从链霉菌属的培养材料中发现了三种新的抗疟原虫化合物,命名为 akedanones A ( 1 )、B ( 2 ) 和 C ( 3 ) 。 K20-0187 从日本山梨县甲府武田采集的土壤样品中分离出来。通过质谱和核磁共振分析,化合物1-3的结构被阐明为具有异戊二烯基和反异戊二烯基的新型2,3-二氢萘醌。化合物1和已知的呋喃萘醌 I ( 4 )对氯喹敏感和氯喹耐药的恶性疟原虫菌株表现出有效的体外抗疟原虫活性,半数最大抑制浓度值范围为 0.06 至 0.3 μM。化合物1和4对药物敏感的啮齿类疟疾伯氏疟原虫N 株也表现出有效的体内抗疟原虫活性,腹膜内给药剂量 5 mg·kg –1天–1 4 次后,抑制率分别为 47.6 和 43.1%天。
更新日期:2024-02-29
中文翻译:

Akedanones A–C,体外和体内 抗疟原虫 2,3-二氢萘醌 由链霉菌产生。 K20-0187
从链霉菌属的培养材料中发现了三种新的抗疟原虫化合物,命名为 akedanones A ( 1 )、B ( 2 ) 和 C ( 3 ) 。 K20-0187 从日本山梨县甲府武田采集的土壤样品中分离出来。通过质谱和核磁共振分析,化合物1-3的结构被阐明为具有异戊二烯基和反异戊二烯基的新型2,3-二氢萘醌。化合物1和已知的呋喃萘醌 I ( 4 )对氯喹敏感和氯喹耐药的恶性疟原虫菌株表现出有效的体外抗疟原虫活性,半数最大抑制浓度值范围为 0.06 至 0.3 μM。化合物1和4对药物敏感的啮齿类疟疾伯氏疟原虫N 株也表现出有效的体内抗疟原虫活性,腹膜内给药剂量 5 mg·kg –1天–1 4 次后,抑制率分别为 47.6 和 43.1%天。



























 京公网安备 11010802027423号
京公网安备 11010802027423号