当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Monitoring of Competitive Coformer Exchange Reaction by 1H MAS Solid-State NMR
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-02-19 , DOI: 10.1021/acs.molpharmaceut.3c01118 Chaithanya Hareendran 1, 2 , Bashir Alsirawan 3 , Anant Paradkar 3 , T. G. Ajithkumar 1, 2
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-02-19 , DOI: 10.1021/acs.molpharmaceut.3c01118 Chaithanya Hareendran 1, 2 , Bashir Alsirawan 3 , Anant Paradkar 3 , T. G. Ajithkumar 1, 2
Affiliation
|
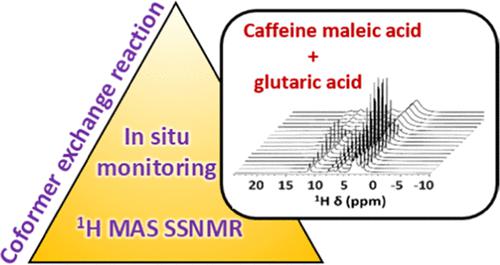
In a competitive coformer exchange reaction, a recent topic of interest in pharmaceutical research, the coformer in a pharmaceutical cocrystal is exchanged with another coformer that is expected to form a cocrystal that is more stable. There will be a competition between coformers to form the most stable product through the formation of hydrogen bonds. This will cause destabilization of the pharmaceutical products during processing or storage. Therefore, it is important to develop a mechanistic understanding of this transformation by monitoring each and every step of the reaction, employing a technique such as 1H nuclear magnetic resonance (NMR). In this study, an in situ monitoring of a coformer exchange reaction is carried out by 1H magic angle spinning (MAS) solid-state NMR (SSNMR) at a spinning frequency of 60 kHz. The changes in caffeine maleic acid cocrystals on addition of glutaric acid and caffeine glutaric cocrystals on addition of maleic acid were monitored. In all of the reactions, it has been observed that caffeine glutaric acid Form I is formed. When glutaric acid was added to 2:1 caffeine maleic acid, the formation of metastable 1:1 caffeine glutaric acid Form I was observed at the start of the experiment, indicating that the centrifugal pressure is enough for the formation. The difference in the end product of the reactions with a similar reaction pathway of 1:1 and 2:1 reactant stoichiometry indicates that a complete replacement of maleic acid has occurred only in the 1:1 stoichiometry of the reactants. The polymorphic transition of caffeine glutaric acid Form II to Form I at higher temperatures was a crucial reason that triggered the exchange of glutaric acid with maleic acid in the reaction of caffeine glutaric acid and maleic acid. Our results are novel since the new reaction pathways in competitive coformer exchange reactions enabled understanding the remarkable role of stoichiometry, polymorphism, temperature, and centrifugal pressure.
中文翻译:

通过 1H MAS 固态 NMR 原位监测竞争性共聚体交换反应
在竞争性共形成物交换反应中,药物共晶中的共形成物与另一种共形成物交换,该反应有望形成更稳定的共晶。竞争性共形成物交换反应是药物研究中最近感兴趣的主题。共形成体之间将存在竞争,通过氢键的形成形成最稳定的产物。这将导致药品在加工或储存过程中不稳定。因此,通过使用1 H 核磁共振 (NMR)等技术监测反应的每一步,对这种转化形成机理理解非常重要。在这项研究中,通过1 H 魔角旋转 (MAS) 固态 NMR (SSNMR) 在 60 kHz 的旋转频率下对共聚体交换反应进行了原位监测。监测咖啡因马来酸共晶体在添加戊二酸时的变化以及咖啡因戊二酸共晶体在添加马来酸时的变化。在所有反应中,观察到形成了咖啡因戊二酸晶型I。当戊二酸添加到2:1咖啡因马来酸中时,在实验开始时观察到亚稳态1:1咖啡因戊二酸I型的形成,表明离心压力足以形成。具有 1:1 和 2:1 反应物化学计量的类似反应途径的反应最终产物的差异表明,仅在 1:1 反应物化学计量中发生了马来酸的完全取代。咖啡因戊二酸II型向I型在较高温度下的多晶型转变是咖啡因戊二酸与马来酸反应中引发戊二酸与马来酸交换的关键原因。我们的结果是新颖的,因为竞争性共聚体交换反应中的新反应途径使我们能够理解化学计量、多态性、温度和离心压力的显着作用。
更新日期:2024-02-19
中文翻译:

通过 1H MAS 固态 NMR 原位监测竞争性共聚体交换反应
在竞争性共形成物交换反应中,药物共晶中的共形成物与另一种共形成物交换,该反应有望形成更稳定的共晶。竞争性共形成物交换反应是药物研究中最近感兴趣的主题。共形成体之间将存在竞争,通过氢键的形成形成最稳定的产物。这将导致药品在加工或储存过程中不稳定。因此,通过使用1 H 核磁共振 (NMR)等技术监测反应的每一步,对这种转化形成机理理解非常重要。在这项研究中,通过1 H 魔角旋转 (MAS) 固态 NMR (SSNMR) 在 60 kHz 的旋转频率下对共聚体交换反应进行了原位监测。监测咖啡因马来酸共晶体在添加戊二酸时的变化以及咖啡因戊二酸共晶体在添加马来酸时的变化。在所有反应中,观察到形成了咖啡因戊二酸晶型I。当戊二酸添加到2:1咖啡因马来酸中时,在实验开始时观察到亚稳态1:1咖啡因戊二酸I型的形成,表明离心压力足以形成。具有 1:1 和 2:1 反应物化学计量的类似反应途径的反应最终产物的差异表明,仅在 1:1 反应物化学计量中发生了马来酸的完全取代。咖啡因戊二酸II型向I型在较高温度下的多晶型转变是咖啡因戊二酸与马来酸反应中引发戊二酸与马来酸交换的关键原因。我们的结果是新颖的,因为竞争性共聚体交换反应中的新反应途径使我们能够理解化学计量、多态性、温度和离心压力的显着作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号