当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Near UV Photodegradation Mechanisms of Amino Acid Excipients: Formation of the Carbon Dioxide Radical Anion from Aspartate and Fe(III)
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.molpharmaceut.3c00893 Yilue Zhang 1 , Yaqi Wu 1 , Christian Schöneich 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.molpharmaceut.3c00893 Yilue Zhang 1 , Yaqi Wu 1 , Christian Schöneich 1
Affiliation
|
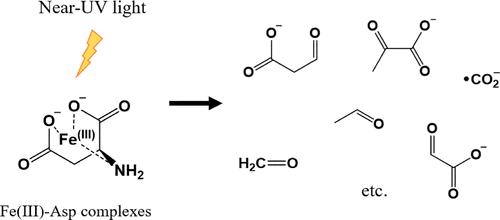
Carbon dioxide radical anion (•CO2–) is a powerful reducing agent that can reduce protein disulfide bonds and convert molecular oxygen to superoxide. Therefore, the generation of •CO2– can be detrimental to pharmaceutical formulations. Iron is among the most prevalent impurities in formulations, where Fe(III) chelates of histidine (His) can produce •CO2– upon exposure to near-UV light (Zhang and Schöneich, Eur. J. Pharm. Biopharm. 2023, 190, 231–241). Here, we monitor by spin-trapping in combination with electron paramagnetic resonance spectroscopy and/or high-performance liquid chromatography–mass spectrometry analysis the photochemical formation of •CO2– for a series of common amino acid excipients, including arginine (Arg), methionine (Met), proline (Pro), glutamic acid (Glu), glycine (Gly), aspartic acid (Asp), and lysine (Lys). Our results indicate that in the presence of Fe(III), Asp, and Glu produce significant yields of •CO2– under photoirradiation with near-UV light. Notably, Asp demonstrates the highest efficiency of •CO2– generation compared with that of the other amino acid excipients. Stable isotope labeling indicates that •CO2– exclusively originates from the α-carboxyl group of Asp. Mechanistic studies reveal two possible pathways for •CO2– formation, which involve either a β-carboxyl radical or an amino radical cation intermediate.
中文翻译:

氨基酸赋形剂的近紫外光降解机制:天冬氨酸和 Fe(III) 形成二氧化碳自由基阴离子
二氧化碳自由基阴离子(· CO 2 –)是一种强还原剂,可以还原蛋白质二硫键,将分子氧转化为超氧化物。因此, • CO 2 –的产生可能对药物配方有害。铁是制剂中最常见的杂质之一,组氨酸 (His) 的 Fe(III) 螯合物在暴露于近紫外光时会产生• CO 2 ( Zhang 和 Schöneich, Eur. J. Pharm. Biopharm . 2023 , 190 ,231-241)。在这里,我们通过自旋捕获结合电子顺磁共振波谱和/或高效液相色谱-质谱分析来监测一系列常见氨基酸赋形剂(包括精氨酸 ( Arg )、蛋氨酸 (Met)、脯氨酸 (Pro)、谷氨酸 (Glu)、甘氨酸 (Gly)、天冬氨酸 (Asp) 和赖氨酸 (Lys)。我们的结果表明,在存在Fe(III)、Asp 和Glu 的情况下,在近紫外光的光照射下,会产生显着的CO 2产量。值得注意的是,与其他氨基酸赋形剂相比,Asp具有最高的CO 2生成效率。稳定同位素标记表明• CO 2 –完全源自 Asp 的 α-羧基。机理研究揭示了CO 2 –形成的两种可能途径,其中涉及β-羧基自由基或氨基自由基阳离子中间体。
更新日期:2024-02-13
中文翻译:

氨基酸赋形剂的近紫外光降解机制:天冬氨酸和 Fe(III) 形成二氧化碳自由基阴离子
二氧化碳自由基阴离子(· CO 2 –)是一种强还原剂,可以还原蛋白质二硫键,将分子氧转化为超氧化物。因此, • CO 2 –的产生可能对药物配方有害。铁是制剂中最常见的杂质之一,组氨酸 (His) 的 Fe(III) 螯合物在暴露于近紫外光时会产生• CO 2 ( Zhang 和 Schöneich, Eur. J. Pharm. Biopharm . 2023 , 190 ,231-241)。在这里,我们通过自旋捕获结合电子顺磁共振波谱和/或高效液相色谱-质谱分析来监测一系列常见氨基酸赋形剂(包括精氨酸 ( Arg )、蛋氨酸 (Met)、脯氨酸 (Pro)、谷氨酸 (Glu)、甘氨酸 (Gly)、天冬氨酸 (Asp) 和赖氨酸 (Lys)。我们的结果表明,在存在Fe(III)、Asp 和Glu 的情况下,在近紫外光的光照射下,会产生显着的CO 2产量。值得注意的是,与其他氨基酸赋形剂相比,Asp具有最高的CO 2生成效率。稳定同位素标记表明• CO 2 –完全源自 Asp 的 α-羧基。机理研究揭示了CO 2 –形成的两种可能途径,其中涉及β-羧基自由基或氨基自由基阳离子中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号