Annals of Nuclear Medicine ( IF 2.6 ) Pub Date : 2024-01-29 , DOI: 10.1007/s12149-023-01895-0 Kentaro Tamura , Ryuichi Nishii , Kotaro Tani , Hiroki Hashimoto , Kazunori Kawamura , Ming-Rong Zhang , Takamasa Maeda , Kana Yamazaki , Tatsuya Higashi , Masahiro Jinzaki
|
Purpose
N-benzyl-N-methyl-2-[7, 8-dihydro-7-(2-[18F] fluoroethyl) -8-oxo-2-phenyl-9H-purin-9-yl] acetamide ([18F] FEDAC) is a novel positron emission tomography (PET) tracer that targets the translocator protein (TSPO; 18 kDa) in the mitochondrial outer membrane, which is known to be upregulated in various diseases such as malignant tumors, neurodegenerative diseases, and neuroinflammation. This study presents the first attempt to use [18F]FEDAC PET/CT and evaluate its biodistribution as well as the systemic radiation exposure to the radiotracer in humans.
Materials and Methods
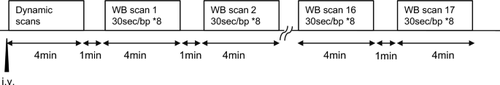
Seventeen whole-body [18F]FEDAC PET/CT (injected dose, 209.1 ± 6.2 MBq) scans with a dynamic scan of the upper abdomen were performed in seven participants. Volumes of interest were assigned to each organ, and a time–activity curve was created to evaluate the biodistribution of the radiotracer. The effective dose was calculated using IDAC-Dose 2.1.
Results
Immediately after the intravenous injection, the radiotracer accumulated significantly in the liver and was subsequently excreted into the gastrointestinal tract through the biliary tract. It also showed high levels of accumulation in the kidneys, but showed minimal migration to the urinary bladder. Thus, the liver was the principal organ that eliminated [18F] FEDAC. Accumulation in the normal brain tissue was minimal. The effective dose estimated from biodistribution in humans was 19.47 ± 1.08 µSv/MBq, and was 3.60 mSV for 185 MBq dose.
Conclusion
[18F]FEDAC PET/CT provided adequate image quality at an acceptable effective dose with no adverse effects. Therefore, [18F]FEDAC may be useful in human TSPO-PET imaging.
中文翻译:

[18F] FEDAC 的首次人体研究:18-kDa 易位蛋白的新型 PET 示踪剂
摘要
目的
N-苄基-N-甲基-2-[7, 8-二氢-7-(2-[ 18 F]氟乙基)-8-氧代-2-苯基-9H-嘌呤-9-基]乙酰胺([ 18 F ] ] FEDAC)是一种新型正电子发射断层扫描(PET)示踪剂,以线粒体外膜中的易位蛋白(TSPO;18 kDa)为目标,已知该蛋白在恶性肿瘤、神经退行性疾病和神经炎症等多种疾病中表达上调。本研究首次尝试使用[ 18 F]FEDAC PET/CT 并评估其生物分布以及人体放射性示踪剂的全身辐射暴露。
材料和方法
对 7 名参与者进行了17 次全身 [ 18 F]FEDAC PET/CT(注射剂量,209.1 ± 6.2 MBq)扫描,并对上腹部进行动态扫描。将感兴趣的体积分配给每个器官,并创建时间-活性曲线来评估放射性示踪剂的生物分布。使用 IDAC-Dose 2.1 计算有效剂量。
结果
静脉注射后,放射性示踪剂立即在肝脏中大量积聚,随后通过胆道排泄到胃肠道。它还在肾脏中显示出高水平的积累,但向膀胱的迁移却很少。因此,肝脏是消除[ 18 F] FEDAC 的主要器官。正常脑组织中的蓄积量极少。根据人体生物分布估计的有效剂量为 19.47 ± 1.08 µSv/MBq,185 MBq 剂量为 3.60 mSV。
结论
[ 18 F]FEDAC PET/CT 在可接受的有效剂量下提供了足够的图像质量,并且没有副作用。因此,[ 18 F]FEDAC可能在人类TSPO-PET成像中有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号