当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantitative analysis of tetrahydrocannabinol isomers and other toxicologically relevant drugs in blood
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2023-12-30 , DOI: 10.1002/dta.3632 Dylan Mantinieks 1, 2 , Matthew Di Rago 1, 2 , Olaf H. Drummer 1, 2 , Linda Glowacki 2 , Jennifer Schumann 1, 2 , Dimitri Gerostamoulos 1, 2
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2023-12-30 , DOI: 10.1002/dta.3632 Dylan Mantinieks 1, 2 , Matthew Di Rago 1, 2 , Olaf H. Drummer 1, 2 , Linda Glowacki 2 , Jennifer Schumann 1, 2 , Dimitri Gerostamoulos 1, 2
Affiliation
|
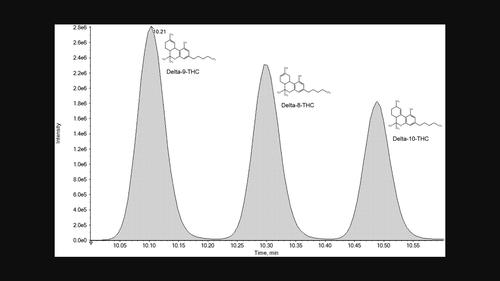
A multi-analyte liquid chromatography-tandem mass spectrometry (LC-MS/MS) method is described, involving the separation of delta-9-tetrahydrocannabinol (delta-9-THC) and delta-8-THC in addition to other commonly encountered drugs and metabolites. Briefly, sample preparation involved an alkaline liquid–liquid extraction (methyl tert-butyl ether) of blood (100 μl). The solvent layer was transferred, evaporated to dryness, reconstituted, and samples then separated on an Agilent Poroshell 120 EC-C18 100 Å (50 mm × 3.0 mm, 2.7 μm) analytical column using a multi-step gradient elution of 50 mM ammonium formate in water (pH 3.5) and 0.1% formic acid in methanol over 14 min. A SCIEX Triple Quad 6500+ system operating in scheduled multiple reaction monitoring and positive electrospray ionization was used for detection. There were no interferences, and matrix effects were generally acceptable (±20% of neat response). Linearity was achieved within the calibration range, including methylamphetamine (MA) (10–1000 ng/ml), 3,4-methylenedioxy-N-methylamphetamine (MDMA) (10–1,000 ng/ml), cocaine (10–1000 ng/ml), and two THC isomers (1–100 ng/ml). Accuracies of MA, MDMA, cocaine, and two THC isomers were 3.6 to 8.9%, −1.2 to 4%, −5.3 to 5.8%, and −11 to 14%, respectively; while precision estimates of the same were 1.6 to 5.4%, 1.7 to 5.3%, 1.2 to 4.5%, and 2 to 10%, respectively. Autosampler stability and dilution integrity were within acceptable limits, and no carryover was detected at the limit of detection. This validated LC-MS/MS method made the routine identification of both delta-9-THC and delta-8-THC in blood possible.
中文翻译:

血液中四氢大麻酚异构体和其他毒理学相关药物的定量分析
描述了一种多分析物液相色谱-串联质谱 (LC-MS/MS) 方法,除了其他常见药物外,还涉及分离 delta-9-四氢大麻酚 (delta-9-THC) 和 delta-8-THC和代谢物。简而言之,样品制备涉及血液(100 μl)的碱性液液萃取(甲基叔丁基醚)。转移溶剂层,蒸发至干,复溶,然后在 Agilent Poroshell 120 EC-C18 100 Å (50 mm × 3.0 mm, 2.7 µm) 分析柱上使用 50 mM 甲酸铵多步梯度洗脱对样品进行分离在水 (pH 3.5) 和 0.1% 甲酸的甲醇溶液中浸泡 14 分钟。使用按计划的多反应监测和正电喷雾电离运行的 SCIEX Triple Quad 6500+ 系统进行检测。没有干扰,基质效应通常可以接受(纯响应的±20%)。在校准范围内实现线性,包括甲基苯丙胺 (MA) (10–1000 ng/ml)、3,4-亚甲基二氧基-N-甲基苯丙胺 (MDMA) (10–1,000 ng/ml)、可卡因 (10–1000 ng/ml) ml) 和两种 THC 异构体 (1–100 ng/ml)。MA、MDMA、可卡因和两种 THC 异构体的准确度分别为 3.6 至 8.9%、-1.2 至 4%、-5.3 至 5.8% 和 -11 至 14%;其精度估计分别为1.6%至5.4%、1.7%至5.3%、1.2%至4.5%和2%至10%。自动进样器的稳定性和稀释完整性在可接受的限度内,并且在检测限度内未检测到残留。这种经过验证的 LC-MS/MS 方法使得血液中 delta-9-THC 和 delta-8-THC 的常规鉴定成为可能。
更新日期:2023-12-31
中文翻译:

血液中四氢大麻酚异构体和其他毒理学相关药物的定量分析
描述了一种多分析物液相色谱-串联质谱 (LC-MS/MS) 方法,除了其他常见药物外,还涉及分离 delta-9-四氢大麻酚 (delta-9-THC) 和 delta-8-THC和代谢物。简而言之,样品制备涉及血液(100 μl)的碱性液液萃取(甲基叔丁基醚)。转移溶剂层,蒸发至干,复溶,然后在 Agilent Poroshell 120 EC-C18 100 Å (50 mm × 3.0 mm, 2.7 µm) 分析柱上使用 50 mM 甲酸铵多步梯度洗脱对样品进行分离在水 (pH 3.5) 和 0.1% 甲酸的甲醇溶液中浸泡 14 分钟。使用按计划的多反应监测和正电喷雾电离运行的 SCIEX Triple Quad 6500+ 系统进行检测。没有干扰,基质效应通常可以接受(纯响应的±20%)。在校准范围内实现线性,包括甲基苯丙胺 (MA) (10–1000 ng/ml)、3,4-亚甲基二氧基-N-甲基苯丙胺 (MDMA) (10–1,000 ng/ml)、可卡因 (10–1000 ng/ml) ml) 和两种 THC 异构体 (1–100 ng/ml)。MA、MDMA、可卡因和两种 THC 异构体的准确度分别为 3.6 至 8.9%、-1.2 至 4%、-5.3 至 5.8% 和 -11 至 14%;其精度估计分别为1.6%至5.4%、1.7%至5.3%、1.2%至4.5%和2%至10%。自动进样器的稳定性和稀释完整性在可接受的限度内,并且在检测限度内未检测到残留。这种经过验证的 LC-MS/MS 方法使得血液中 delta-9-THC 和 delta-8-THC 的常规鉴定成为可能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号