Neuron ( IF 16.2 ) Pub Date : 2023-12-22 , DOI: 10.1016/j.neuron.2023.11.024 Jacqueline E. Paniccia , Kelsey M. Vollmer , Lisa M. Green , Roger I. Grant , Kion T. Winston , Sophie Buchmaier , Annaka M. Westphal , Rachel E. Clarke , Elizabeth M. Doncheck , Bogdan Bordieanu , Logan M. Manusky , Michael R. Martino , Amy L. Ward , Jennifer A. Rinker , Jacqueline F. McGinty , Michael D. Scofield , James M. Otis
|
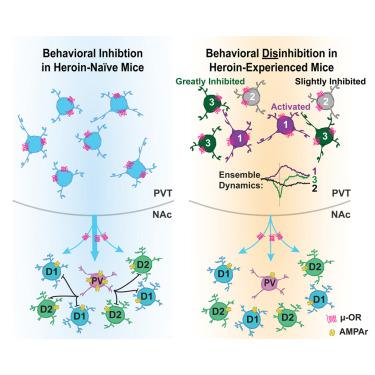
Lack of behavioral suppression typifies substance use disorders, yet the neural circuit underpinnings of drug-induced behavioral disinhibition remain unclear. Here, we employ deep-brain two-photon calcium imaging in heroin self-administering mice, longitudinally tracking adaptations within a paraventricular thalamus to nucleus accumbens behavioral inhibition circuit from the onset of heroin use to reinstatement. We find that select thalamo-accumbal neuronal ensembles become profoundly hypoactive across the development of heroin seeking and use. Electrophysiological experiments further reveal persistent adaptations at thalamo-accumbal parvalbumin interneuronal synapses, whereas functional rescue of these synapses prevents multiple triggers from initiating reinstatement of heroin seeking. Finally, we find an enrichment of μ-opioid receptors in output- and cell-type-specific paraventricular thalamic neurons, which provide a mechanism for heroin-induced synaptic plasticity and behavioral disinhibition. These findings reveal key circuit adaptations that underlie behavioral disinhibition in opioid dependence and further suggest that recovery of this system would reduce relapse susceptibility.
中文翻译:

室旁丘脑累积行为抑制回路的恢复可防止海洛因寻求的恢复
缺乏行为抑制是物质使用障碍的典型特征,但药物引起的行为去抑制的神经回路基础仍不清楚。在这里,我们在海洛因自我给药小鼠中采用深脑双光子钙成像,纵向追踪从海洛因使用开始到恢复的室旁丘脑到伏核行为抑制回路的适应。我们发现,在海洛因寻求和使用的过程中,特定的丘脑累及神经元群变得极度活跃。电生理学实验进一步揭示了丘脑-累加小白蛋白神经元间突触的持续适应,而这些突触的功能性拯救可防止多种触发因素启动海洛因寻求的恢复。最后,我们发现输出和细胞类型特异性的室旁丘脑神经元中μ阿片受体富集,这为海洛因诱导的突触可塑性和行为去抑制提供了机制。这些发现揭示了阿片类药物依赖性行为去抑制背后的关键回路适应,并进一步表明该系统的恢复将降低复发的易感性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号