European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2023-10-08 , DOI: 10.1016/j.ejmech.2023.115836 Silong Zhang 1 , Yu Zhang 2 , Ziwei Wang 2 , Luolong Qing 2 , Shaojuan Fu 3 , Juan Xu 4 , Yuanyuan Li 5 , Huaxiang Fang 6 , Huan He 1
|
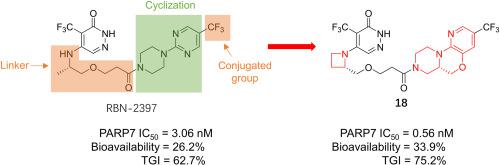
PARP7 has emerged as a promising anti-tumor target due to its crucial roles in nucleic acid sensing and immune regulation. Herein, we explored the structural-activity relationship of tricyclic PARP7 inhibitors containing a hexahydropyrazino[1,2-d]pyrido[3,2-b][1,4]oxazine motif. The effects of the chirality of the fused rings, the group conjugated to the fused rings, and the size of the linker on PARP7 inhibition were fully investigated. Our work leads to the discovery of an extremely potent and orally-bioavailable PARP7 inhibitor, namely 18 (PARP7 inhibition IC50 = 0.56 nM), for efficacious treatment of lung cancer in vivo. Notably, 18 showed acceptable bioavailability in ICR mice (F = 33.9%) and Beagle dogs (F = 45.2%). Further investigation of ADME-T properties suggested that 18 has the potential to be developed as a candidate drug molecule for PARP7-sensitive tumors.
中文翻译:

探索六氢吡嗪并[1,2-d]吡啶并[3,2-b][1,4]恶嗪衍生物作为有效且口服生物可利用的 PARP7 抑制剂的结构-活性关系
PARP7因其在核酸传感和免疫调节中的关键作用而成为一种有前途的抗肿瘤靶点。在此,我们探讨了含有六氢吡嗪并[1,2-d]吡啶并[3,2-b][1,4]恶嗪基序的三环PARP7抑制剂的结构-活性关系。充分研究了稠合环的手性、与稠合环缀合的基团以及接头的大小对 PARP7 抑制的影响。我们的工作发现了一种极其有效且可口服生物利用的 PARP7 抑制剂,即18(PARP7 抑制 IC 50 = 0.56 nM),可在体内 有效治疗肺癌。值得注意的是, 18在 ICR 小鼠(F = 33.9%) 和 Beagle 犬 (F = 45.2%) 中表现出可接受的生物利用度。对 ADME-T 特性的进一步研究表明18有潜力被开发为 PARP7 敏感肿瘤的候选药物分子。


































 京公网安备 11010802027423号
京公网安备 11010802027423号