Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Study of Silica Nanoparticles and CO2-Switchable Surfactants at an Oil–Water Interface
Langmuir ( IF 3.9 ) Pub Date : 2023-07-31 , DOI: 10.1021/acs.langmuir.3c00949 Tong Meng , Zhen Zhao , Guangyong Li , Jun Li , Hui Yan
Langmuir ( IF 3.9 ) Pub Date : 2023-07-31 , DOI: 10.1021/acs.langmuir.3c00949 Tong Meng , Zhen Zhao , Guangyong Li , Jun Li , Hui Yan
|
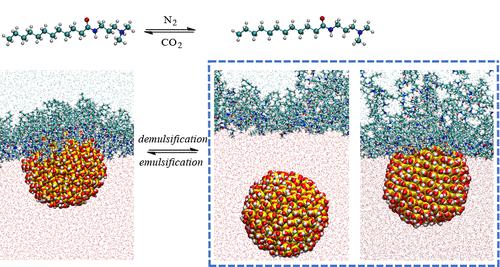
Adsorbing CO2-sensitive surfactants on the surface of nanoparticles is an important strategy for preparing stimuli-responsive Pickering emulsions. However, the microscopic mechanisms are still limited, owing to a lack of intuitive understanding at the molecular level on the interactions between nanoparticle and switchable surfactants at the oil–water interface. We employed the molecular dynamics (MD) simulations to explore the mechanism behind the reversible emulsification/demulsification of a Pickering emulsion stabilized by silica nanoparticles (NPs) and CO2-switchable surfactants, named N-(3-(dimethylamino)propyl)alkyl amide (CPMA). MD results show that the protonated surfactant CPMAH+ has strong hydrophilicity, forming an adsorption layer at the oil–water interface. The ionic surfactants can be tightly adsorbed on NP surface through electrostatic interactions. Thus, the formed colloid particle has both hydrophobic and hydrophilic properties, which is a key factor in stabilizing emulsion. When CPMAH+ molecules were deprotonated to CPMA, the hydration activity of the headgroups reduced greatly, inducing a mixture with oil molecules. There are still a certain number of CPMA molecules residing at the oil–water interface due to the hydrophilic amine groups. The results from repeated simulations show that NP can either stay in the water phase or locate at the interface. Even NP was finally adsorbed on the interface and combined with CPMA or oil molecules, the adsorption configuration of CPMA on the NP surface was essentially different from that of CPMAH+. The potential of mean force confirmed that the combination between NP and CPMA is quite unstable due to the disappearance of electrostatic attraction. Different binding configurations and stability between NP and CPMA or CPMAH+ were the fundamental reason for the reversible emulsification/demulsification of Pickering emulsion.
中文翻译:

二氧化硅纳米颗粒和 CO2 可转换表面活性剂在油水界面的分子动力学研究
在纳米颗粒表面吸附CO 2敏感表面活性剂是制备刺激响应性Pickering乳液的重要策略。然而,由于在分子水平上缺乏对油水界面纳米粒子和可转换表面活性剂之间相互作用的直观理解,微观机制仍然有限。我们采用分子动力学 (MD) 模拟来探索由二氧化硅纳米颗粒 (NP) 和 CO 2 可切换表面活性剂(称为 N -(3-(二甲基氨基)丙基)烷基酰胺)稳定的 Pickering 乳液的可逆乳化/破乳背后的机制(CPMA)。MD结果表明,质子化表面活性剂CPMAH +具有较强的亲水性,在油水界面形成吸附层。离子表面活性剂可以通过静电相互作用紧密吸附在纳米粒子表面。因此,形成的胶体颗粒同时具有疏水性和亲水性,这是稳定乳液的关键因素。当CPMAH +分子去质子化为CPMA时,头基的水合活性大大降低,诱导与油分子的混合物。由于具有亲水性胺基,仍有一定数量的CPMA分子驻留在油水界面上。重复模拟的结果表明,NP可以停留在水相中,也可以位于界面处。即使NP最终吸附在界面上并与CPMA或油分子结合,CPMA在NP表面的吸附构型与CPMAH +的吸附构型也有本质的不同。平均力势证实,由于静电引力的消失,NP和CPMA之间的结合相当不稳定。NP与CPMA或CPMAH +之间不同的结合构型和稳定性是Pickering乳液可逆乳化/破乳的根本原因。
更新日期:2023-07-31
中文翻译:

二氧化硅纳米颗粒和 CO2 可转换表面活性剂在油水界面的分子动力学研究
在纳米颗粒表面吸附CO 2敏感表面活性剂是制备刺激响应性Pickering乳液的重要策略。然而,由于在分子水平上缺乏对油水界面纳米粒子和可转换表面活性剂之间相互作用的直观理解,微观机制仍然有限。我们采用分子动力学 (MD) 模拟来探索由二氧化硅纳米颗粒 (NP) 和 CO 2 可切换表面活性剂(称为 N -(3-(二甲基氨基)丙基)烷基酰胺)稳定的 Pickering 乳液的可逆乳化/破乳背后的机制(CPMA)。MD结果表明,质子化表面活性剂CPMAH +具有较强的亲水性,在油水界面形成吸附层。离子表面活性剂可以通过静电相互作用紧密吸附在纳米粒子表面。因此,形成的胶体颗粒同时具有疏水性和亲水性,这是稳定乳液的关键因素。当CPMAH +分子去质子化为CPMA时,头基的水合活性大大降低,诱导与油分子的混合物。由于具有亲水性胺基,仍有一定数量的CPMA分子驻留在油水界面上。重复模拟的结果表明,NP可以停留在水相中,也可以位于界面处。即使NP最终吸附在界面上并与CPMA或油分子结合,CPMA在NP表面的吸附构型与CPMAH +的吸附构型也有本质的不同。平均力势证实,由于静电引力的消失,NP和CPMA之间的结合相当不稳定。NP与CPMA或CPMAH +之间不同的结合构型和稳定性是Pickering乳液可逆乳化/破乳的根本原因。























































 京公网安备 11010802027423号
京公网安备 11010802027423号