Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2023-07-07 , DOI: 10.1016/j.molstruc.2023.136135 Riya Singh , Poonam Rawat , Anshu Gautam , Mukesh Kumar , Poonam Bharati , Shipra Gautam , Anant Ram , Prakash , Amul Darwari , Norma RosarioFlores Holguín , R.N. Singh
|
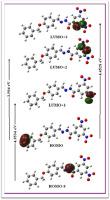
The 4–(Benzyloxy) benzaldehyde–3, 5–dinitrobenzohydrazone (3) has been synthesized spectroscopically, characterized and its antitubercularactivity has been evaluated. All the quantum chemical calculations have been carried out using DFT level of theory, B3LYP functional and 6-311++G(d,p) basis set. The presence of chemical shift of azomethine proton singlet at 8.246 ppm and absence of singlet at 3.1 ppm in experimental 1H NMR spectrum indicated that the molecule exits in hydrazide-hydrazone form. The N–H stretching band in the FT-IR spectrum of (3) show Davidov coupling between the neighboring units and observed in the region 3471–3226 cm–1. NBO analysis confirms the various intramolecular charge transfer from donor to acceptor and stabilize the molecule upto∼24.65 kcal/mol due to π→π* and upto∼16.60 kcal/mol due to n→π* interaction. The maximum values of the electrophilic reactivity descriptors at C14 indicate that this site is more prone to nucleophilic attack. The local reactivity descriptors for (3) favor the formation of oxadiazoline, thiadiazoline, thiazolidinones, azetidinonesetc by attack of nucleophilic part of the dipolar reagent on C14 site of C14=N15 bond. The calculated β0 (14.3915 × 10–31 esu) value indicates it's suitability for NLO application. The compound (3) was evaluated for their antitubercular potential against Mycobacterium tuberculosis H37Rv using microplate alamar blue assay (MABA). Docking study of (3) showed binding conformations and affinities with the target protein through hydrogen bonding.
中文翻译:

4-(苄氧基)苯甲醛-3,5-二硝基苯腙的合成、抗分枝杆菌活性评价及分子对接:实验与理论相结合的方法
4-(苄氧基)苯甲醛-3, 5-二硝基苯腙 (3) 已通过光谱合成、表征并评估其抗结核活性。所有量子化学计算均使用DFT水平的理论、B3LYP泛函和6-311++G(d,p)基组进行。实验1 H NMR 谱中 8.246 ppm 处存在偶氮甲碱质子单线态化学位移,而 3.1 ppm 处不存在单线态,表明该分子以酰肼-腙形式存在。(3) 的 FT-IR 光谱中的 N-H 伸缩带显示了相邻单元之间的 Davidov 耦合,并在 3471-3226 cm –1区域观察到。NBO 分析证实了从供体到受体的各种分子内电荷转移,并将分子稳定到~24.65 kcal/mol,这是由于由于n→π * 相互作用, π→π * 和高达∼16.60 kcal/mol 。C14 处亲电子反应性描述符的最大值表明该位点更容易受到亲核攻击。(3) 的局部反应性描述符有利于通过偶极试剂的亲核部分攻击 C14=N15 键的 C14 位点形成恶二唑啉、噻二唑啉、噻唑烷酮、氮杂环丁酮等。计算出的β 0 (14.3915 × 10 –31 esu) 值表明其适合NLO 应用。评估了化合物 (3) 对抗结核分枝杆菌H 37 R v的抗结核潜力使用微孔板阿尔玛蓝测定法 (MABA)。(3)的对接研究显示了通过氢键与目标蛋白的结合构象和亲和力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号