Current Opinion in Immunology ( IF 7 ) Pub Date : 2023-06-07 , DOI: 10.1016/j.coi.2023.102346 Reem Satti 1 , Jack L Morley 1 , Louise H Boyle 1
|
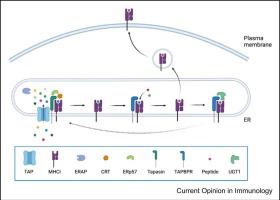
Since the discovery of Transporter associated with antigen processing-binding protein-related (TAPBPR) over two decades ago, extensive studies have explored its function in the context of the major histocompatibility complex class-I (MHC-I) antigen processing and presentation pathway. As a chaperone and peptide editor, TAPBPR was recently revealed to have overlapping structural features when resolved with peptide-receptive MHC-I molecules compared with the two newly solved tapasin:MHC-I structures. Despite this, the two chaperones seem to have a unique criteria for loading high-affinity peptides on MHC-I molecules. Yet, the mechanism of action of how TAPBPR creates its distinct filter in cargo selection for peptide-receptive MHC-I molecules continues to be a subject of debate.
中文翻译:

进入凹槽!TAPBPR对货物选择的影响
自从二十多年前发现与抗原加工结合蛋白相关 (TAPBPR) 相关的转运蛋白以来,广泛的研究探索了其在主要组织相容性复合物 I 类 (MHC-I) 抗原加工和呈递途径中的功能。作为分子伴侣和肽编辑器,最近发现,与两个新解析的 tapasin:MHC-I 结构相比,当用肽受体 MHC-I 分子解析时,TAPBPR 具有重叠的结构特征。尽管如此,这两个伴侣似乎具有在 MHC-I 分子上加载高亲和力肽的独特标准。然而,TAPBPR 如何在肽受体 MHC-I 分子的货物选择中创建其独特的过滤器的作用机制仍然是一个争论的话题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号