Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-06-07 , DOI: 10.1016/j.seppur.2023.124279 Ashish Gautam , Monoj Kumar Mondal
|
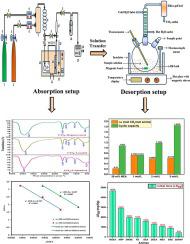
This research is necessary to provide a potential aqueous amine blend solvent for industrial application to help in overcoming the challenges of drastic climate change. The performance of a novel aqueous amine blend of 2-(butylamino)ethanol (BAE) and 2-Dimethylaminoethanol (DMAE) in terms of CO2 absorption and desorption was investigated. This study focused on aspects such as equilibrium CO2 loading, absorption capacity, empirical modeling, cyclic equilibrium CO2 loading, cyclic capacity, heat duty, regeneration efficiency, pH effect, heat of absorption, toxicity assessment, 13C NMR and FTIR speciation, response surface methodology (RSM) modeling, and optimization. The entire CO2 absorption experiment was performed in the temperature (T) ranging from 298.15 to 333.15 K, CO2 partial pressure () ranged from 10.13 to 25.33 kPa, mole fraction of BAE () changed from 0.05 to 0.20, and solution concentration (C) varied from 1 to 3 mol/L. Desorption experiments were carried out at a constant temperature of 393.15 K and a constant pressure of 25.33 kPa. At T = 313.15 K, = 25.33 kPa, = 0.20, and C = 1 mol/L, CO2 absorption experiments yielded a maximum equilibrium CO2 loading of 0.9365 mol CO2/mol amine. At C = 3 mol/L, this novel blend exhibited 71.23 % higher cyclic capacity than conventional 30 wt% monoethanolamine (MEA). Heat duty and regeneration efficiency of 3 mol/L solution were found to be 112.96 kJ/mol CO2 and 83.27 %, respectively. This amine blend's heat of CO2 absorption was determined to be −72.74 kJ/mol. RSM predicted optimum equilibrium CO2 loading of 0.829265 mol CO2/mol amine at T = 306.90 K, = 21.22 kPa, = 0.16, and C = 1.5 mol/L.
中文翻译:

用于 CO2 捕获的 2-(丁基氨基) 乙醇和 2-二甲氨基乙醇的新型水性胺混合物:平衡 CO2 负载、RSM 优化、解吸研究、表征和毒性评估
这项研究对于为工业应用提供潜在的水性胺混合溶剂以帮助克服剧烈气候变化的挑战是必要的。研究了2-(丁基氨基)乙醇 (BAE) 和 2-二甲氨基乙醇 (DMAE) 的新型水性胺混合物在 CO 2吸收和解吸方面的性能。本研究重点关注平衡CO 2负载、吸收能力、经验模型、循环平衡CO 2负载、循环容量、热负荷、再生效率、pH效应、吸收热、毒性评估、13 C NMR和FTIR形态形成等方面,响应面法 (RSM) 建模和优化。整个CO 2吸收实验在温度(T)范围为298.15至333.15 K、CO 2分压()范围为 10.13 至 25.33 kPa,BAE 的摩尔分数()从0.05变化到0.20,溶液浓度(C)从1到3mol/L变化。解吸实验在393.15 K恒温、25.33 kPa恒压下进行。在 T = 313.15 K 时, = 25.33 kPa, = 0.20,并且C = 1 mol/L,CO 2吸收实验产生0.9365 mol CO 2 /mol胺的最大平衡CO 2负载。当 C = 3 mol/L 时,这种新型混合物的循环容量比传统的 30 wt% 单乙醇胺 (MEA) 高出 71.23%。3 mol/L 溶液的热负荷和再生效率分别为112.96 kJ/mol CO 2和83.27 %。该胺混合物的CO 2吸收热测定为-72.74 kJ/mol。RSM 预测 T = 306.90 K 时的最佳平衡 CO 2负载量为 0.829265 mol CO 2 /mol 胺, = 21.22 kPa, = 0.16,C = 1.5 mol/L。











































 京公网安备 11010802027423号
京公网安备 11010802027423号