Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-06-05 , DOI: 10.1016/j.seppur.2023.124200 Young-Ho Cho , Masoud Mofarahi , Kyung-Min Kim , Chang-Ha Lee
|
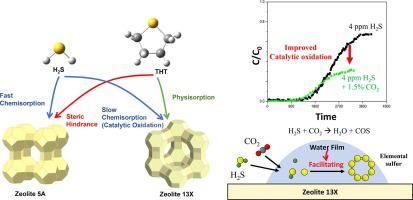
Desulfurization of ultra-low sulfur species remaining in natural gas (NG) is important because of its critical effects on both chemical and clean energy applications. Environmental regulations and fuel cell guidelines require NG with a sulfur content of ppm or less. In this study, the adsorptive behavior of representative trace-level sulfur compounds in CH4 balance, H2S, and tetrahydrothiophene (THT) at 4 and 50 ppm, was investigated on zeolite 5A and 13X pellets. Breakthrough experiments were conducted in various binary systems (one sulfur compound in CH4), and surface analysis of the used zeolites was performed to elucidate the adsorption mechanism. The influence of the CO2 impurity level in CH4 on sulfur removal was also investigated in ternary systems (one sulfur compound with 1.5 % CO2 in a CH4 balance). The main pathway of H2S adsorption on zeolite 13X was catalytic oxidation, whereas it was presumed to be dissociative adsorption on zeolite 5A. In contrast, physisorption was dominant for THT removal in both zeolites. The lower contribution of chemisorption in THT led to less adsorptive amount than that in H2S removal at ultra-low concentrations. Furthermore, the molecular structure of THT (higher kinetic diameter) resulted in a faster breakthrough for zeolite 5A than for zeolite 13X. Therefore, more amount of H2S was removed than THT in both zeolites, and zeolite 13X demonstrated better performance for both sulfur compounds than zeolite 5A. Notably, the presence of 1.5 % CO2 in the feed promotes the catalytic oxidation of H2S.
中文翻译:

在沸石 5A 和 13X 颗粒上吸附去除含和不含 CO2 的 CH4 中的超低浓度 H2S 和 THT
天然气 (NG) 中残留的超低硫物质的脱硫非常重要,因为它对化学和清洁能源应用都有关键影响。环境法规和燃料电池指南要求 NG 的硫含量为 ppm 或更低。在这项研究中,研究了在 4 和 50 ppm 的CH 4平衡、H 2 S 和四氢噻吩 (THT)中代表性痕量硫化合物在沸石 5A 和 13X 颗粒上的吸附行为。在各种二元系统(CH 4中的一种硫化合物)中进行了突破性实验,并对使用过的沸石进行了表面分析以阐明吸附机理。CH 4中CO 2杂质水平的影响还研究了三元系统( CH 4平衡中含有 1.5% CO 2的一种硫化合物)对脱硫的影响。H 2 S在13X沸石上的吸附主要途径是催化氧化,而在5A沸石上推测为解离吸附。相反,在两种沸石中,物理吸附对于去除 THT 占主导地位。THT 中化学吸附的较低贡献导致在超低浓度下吸附量低于 H 2 S 去除。此外,THT 的分子结构(更高的动力学直径)导致沸石 5A 比沸石 13X 更快突破。因此,更多的 H 2在两种沸石中,S 都比 THT 去除了,沸石 13X 对两种含硫化合物的性能均优于沸石 5A。值得注意的是,进料中1.5% CO 2的存在促进了 H 2 S的催化氧化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号