当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unexpected Nucleophile Masking in Acyl Transfer to Sterically Crowded and Conformationally Restricted Galactosides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-03 , DOI: 10.1021/acs.joc.3c00878 Yonatan Sukhran 1 , Israel Alshanski 1 , Ofer Filiba 2 , Megan J Mackintosh 2 , Igor Schapiro 2 , Mattan Hurevich 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-03 , DOI: 10.1021/acs.joc.3c00878 Yonatan Sukhran 1 , Israel Alshanski 1 , Ofer Filiba 2 , Megan J Mackintosh 2 , Igor Schapiro 2 , Mattan Hurevich 1
Affiliation
|
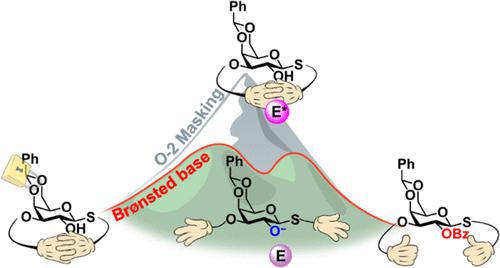
Design and synthesis of orthogonally protected monosaccharide building blocks are crucial for the preparation of well-defined oligosaccharides in a stereo- and regiocontrolled manner. Selective introduction of protecting groups to partially protected monosaccharides is nontrivial due to the often unpredictable electronic, steric, and conformational effects of the substituents. Abolished reactivity toward a commonly used Lewis base-catalyzed acylation of O-2 was observed in conformationally restricted 4,6-O-benzylidene-3-O-Nap galactoside. Investigation of analogous systems, crystallographic characterization, and quantum chemical calculations highlighted the overlooked conformational and steric considerations, the combination of which produces a unique passivity of the 2-OH nucleophile. Evaluating the role of electrophile counterion and auxiliary base in the acylation of the sterically crowded and conformationally restricted galactoside system revealed an alternative Brønsted base-driven reaction pathway via nucleophilic activation. Insights gained from this model system were utilized to access the target galactoside intermediate within the envisioned synthetic route. The acylation strategy described herein can be implemented in future syntheses of key monomeric building blocks with unique protecting group hierarchies.
中文翻译:

酰基转移到空间拥挤和构象限制的半乳糖苷中意外的亲核基掩蔽
正交保护单糖结构单元的设计和合成对于以立体和区域控制方式制备明确的寡糖至关重要。由于取代基的电子、空间和构象效应通常是不可预测的,因此向部分受保护的单糖选择性引入保护基团并非易事。在构象限制的 4,6- O-亚苄基-3- O中观察到常用路易斯碱催化的O -2 酰化的反应性被取消-午睡半乳糖苷。对类似系统、晶体学表征和量子化学计算的研究强调了被忽视的构象和空间考虑因素,它们的组合产生了 2- OH亲核试剂的独特钝性。评估亲电抗衡离子和辅助碱在空间拥挤和构象限制的半乳糖苷系统酰化中的作用揭示了另一种通过亲核活化的布朗斯台德碱驱动反应途径。利用从该模型系统获得的见解来获取设想的合成路线中的目标半乳糖苷中间体。本文描述的酰化策略可以在具有独特保护基团层次结构的关键单体构建块的未来合成中实施。
更新日期:2023-06-03
中文翻译:

酰基转移到空间拥挤和构象限制的半乳糖苷中意外的亲核基掩蔽
正交保护单糖结构单元的设计和合成对于以立体和区域控制方式制备明确的寡糖至关重要。由于取代基的电子、空间和构象效应通常是不可预测的,因此向部分受保护的单糖选择性引入保护基团并非易事。在构象限制的 4,6- O-亚苄基-3- O中观察到常用路易斯碱催化的O -2 酰化的反应性被取消-午睡半乳糖苷。对类似系统、晶体学表征和量子化学计算的研究强调了被忽视的构象和空间考虑因素,它们的组合产生了 2- OH亲核试剂的独特钝性。评估亲电抗衡离子和辅助碱在空间拥挤和构象限制的半乳糖苷系统酰化中的作用揭示了另一种通过亲核活化的布朗斯台德碱驱动反应途径。利用从该模型系统获得的见解来获取设想的合成路线中的目标半乳糖苷中间体。本文描述的酰化策略可以在具有独特保护基团层次结构的关键单体构建块的未来合成中实施。











































 京公网安备 11010802027423号
京公网安备 11010802027423号