Separation and Purification Technology ( IF 8.6 ) Pub Date : 2023-06-02 , DOI: 10.1016/j.seppur.2023.124239 Shuyao Wang , Zhenhao Tong , Weijia An , Wenquan Cui , Jinshan Hu
|
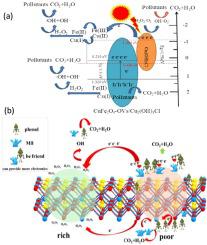
To improve the synergistic degradation activity of photocatalysis-Fenton, CuFe2O4-OVs/Cu2(OH)3Cl composites with two-electron centers were prepared by citric acid complexation and impregnation. Driven by visible light irradiation, a phenol-promoted cooperative degradation system was constructed to degrade methylene blue (MB), which could degrade 93.00% of MB and 100% of phenol within 90 min. The degradation rate constants for MB were 40.80 and 4.35 times higher than those for photocatalysis and Fenton reaction, respectively. Interestingly, the degradation efficiency of MB was doubled when phenol was present in the degradation system, and phenol was also completely degraded. Such excellent improvement in degradation activity is ascribed to (1) the photoassisted valence state conversion of Cu and Fe promotes the photocatalysis-Fenton cycle; (2) In the two-electron center, oxygen vacancies act as electron-rich centers to adsorb and activate H2O2 to generate •OH and consume electrons, while the σ-Cu-ligand complexes formed by Cu(II) and phenol in electron-poor centers are easily attacked by H2O2; (3) phenol acts as an electron donor to promote Cu(II) to capture electrons to achieve rapid degradation of organic contaminants. Based on the analysis of the active species, and degradation intermediates, a synergistic degradation mechanism was proposed, which provides a reference for the efficient degradation of mixed contaminants.
中文翻译:

苯酚辅助光催化-Fenton降解MB的机理:双电子中心与混合污染物的配位
为提高光催化-Fenton的协同降解活性,采用柠檬酸络合浸渍法制备了双电子中心CuFe 2 O 4 -OVs/Cu 2 (OH) 3 Cl复合材料。在可见光照射的驱动下,构建了苯酚促进的协同降解系统来降解亚甲蓝(MB),可在90分钟内降解93.00%的MB和100%的苯酚。MB 的降解速率常数分别是光催化的 40.80 和 4.35 倍,分别为芬顿反应。有趣的是,当降解体系中存在苯酚时,MB 的降解效率提高了一倍,并且苯酚也被完全降解。如此出色的降解活性提高归因于(1)Cu和Fe的光辅助价态转换促进了光催化-芬顿循环;(2) 在双电子中心,氧空位作为富电子中心吸附和活化H 2 O 2生成•OH并消耗电子,而Cu(II)和苯酚形成的σ-Cu-配体络合物在缺电子中心很容易被 H 2 O 2攻击; (3)苯酚作为电子供体促进Cu(II)捕获电子实现有机污染物的快速降解。基于对活性物种和降解中间体的分析,提出了协同降解机制,为混合污染物的高效降解提供了参考。



























 京公网安备 11010802027423号
京公网安备 11010802027423号