当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Photochemical Properties of Fluorescent Metabolites Generated from Fluorinated Benzoylmenadiones in Living Cells
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00620 Nathan Trometer 1 , Bogdan Cichocki 1 , Quentin Chevalier 2 , Jérémy Pécourneau 1 , Jean-Marc Strub 3 , Andréa Hemmerlin 2 , Alexandre Specht 4 , Elisabeth Davioud-Charvet 1 , Mourad Elhabiri 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00620 Nathan Trometer 1 , Bogdan Cichocki 1 , Quentin Chevalier 2 , Jérémy Pécourneau 1 , Jean-Marc Strub 3 , Andréa Hemmerlin 2 , Alexandre Specht 4 , Elisabeth Davioud-Charvet 1 , Mourad Elhabiri 1
Affiliation
|
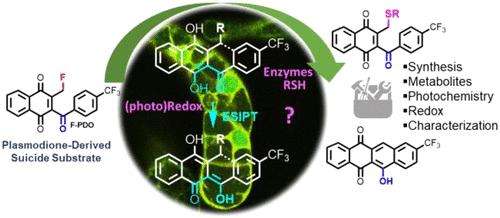
This work describes the reactivity and properties of fluorinated derivatives (F-PD and F-PDO) of plasmodione (PD) and its metabolite, the plasmodione oxide (PDO). Introduction of a fluorine atom on the 2-methyl group markedly alters the redox properties of the 1,4-naphthoquinone electrophore, making the compound highly oxidizing and particularly photoreactive. A fruitful set of analytical methods (electrochemistry, absorption and emission spectrophotometry, and HRMS-ESI) have been used to highlight the products resulting from UV photoirradiation in the absence or presence of selected nucleophiles. With F-PDO and in the absence of nucleophile, photoreduction generates a highly reactive ortho-quinone methide (o-QM) capable of leading to the formation of a homodimer. In the presence of thiol nucleophiles such as β-mercaptoethanol, which was used as a model, o-QMs are continuously regenerated in sequential photoredox reactions generating mono- or disulfanylation products as well as various unreported sulfanyl products. Besides, these photoreduced adducts derived from F-PDO are characterized by a bright yellowish emission due to an excited-state intramolecular proton transfer (ESIPT) process between the dihydronapthoquinone and benzoyl units. In order to evidence the possibility of an intramolecular coupling of the o-QM intermediate, a synthetic route to the corresponding anthrones is described. Tautomerization of the targeted anthrones occurs and affords highly fluorescent stable hydroxyl-anthraquinones. Although probable to explain the intense visible fluorescence emission also observed in tobacco BY-2 cells used as a cellular model, these coupling products have never been observed during the photochemical reactions performed in this study. Our data suggest that the observed ESIPT-induced fluorescence most likely corresponds to the generation of alkylated products through reduction species, as demonstrated with the β-mercaptoethanol model. In conclusion, F-PDO thus acts as a novel (pro)-fluorescent probe for monitoring redox processes and protein alkylation in living cells.
中文翻译:

活细胞中氟化苯甲甲萘醌产生的荧光代谢物的合成和光化学性质
这项工作描述了等离子体二酮 ( PD ) 及其代谢物等离子体二酮氧化物 ( PDO ) 的氟化衍生物( F-PD和F-PDO )的反应性和性质。在 2-甲基上引入氟原子显着改变了 1,4-萘醌电泳剂的氧化还原性质,使该化合物具有高度氧化性且特别具有光反应性。一套卓有成效的分析方法(电化学、吸收和发射分光光度法以及 HRMS-ESI)已用于突出显示在不存在或存在选定亲核试剂的情况下紫外光照射产生的产物。使用F-PDO在不存在亲核试剂的情况下,光还原会生成高反应性的邻醌甲基化物 ( o -QM),能够导致同型二聚体的形成。在硫醇亲核试剂(例如β-巯基乙醇)存在下(用作模型), o -QM 在连续的光氧化还原反应中不断再生,产生单或二硫酰化产物以及各种未报道的硫酰基产物。此外,这些源自F-PDO的光还原加合物的特点是由于二氢萘醌和苯甲酰基单元之间的激发态分子内质子转移 (ESIPT) 过程而发出明亮的黄色发射光。为了证明o -QM中间体分子内偶联的可能性,描述了相应蒽酮的合成路线。目标蒽发生互变异构并提供高度荧光稳定的羟基蒽醌。 尽管可以解释在用作细胞模型的烟草 BY-2 细胞中观察到的强烈可见荧光发射,但在本研究中进行的光化学反应期间从未观察到这些偶联产物。我们的数据表明,观察到的 ESIPT 诱导的荧光很可能对应于通过还原物质生成烷基化产物,如 β-巯基乙醇模型所证明的那样。总之, F-PDO作为一种新型(前)荧光探针,用于监测活细胞中的氧化还原过程和蛋白质烷基化。
更新日期:2023-06-02
中文翻译:

活细胞中氟化苯甲甲萘醌产生的荧光代谢物的合成和光化学性质
这项工作描述了等离子体二酮 ( PD ) 及其代谢物等离子体二酮氧化物 ( PDO ) 的氟化衍生物( F-PD和F-PDO )的反应性和性质。在 2-甲基上引入氟原子显着改变了 1,4-萘醌电泳剂的氧化还原性质,使该化合物具有高度氧化性且特别具有光反应性。一套卓有成效的分析方法(电化学、吸收和发射分光光度法以及 HRMS-ESI)已用于突出显示在不存在或存在选定亲核试剂的情况下紫外光照射产生的产物。使用F-PDO在不存在亲核试剂的情况下,光还原会生成高反应性的邻醌甲基化物 ( o -QM),能够导致同型二聚体的形成。在硫醇亲核试剂(例如β-巯基乙醇)存在下(用作模型), o -QM 在连续的光氧化还原反应中不断再生,产生单或二硫酰化产物以及各种未报道的硫酰基产物。此外,这些源自F-PDO的光还原加合物的特点是由于二氢萘醌和苯甲酰基单元之间的激发态分子内质子转移 (ESIPT) 过程而发出明亮的黄色发射光。为了证明o -QM中间体分子内偶联的可能性,描述了相应蒽酮的合成路线。目标蒽发生互变异构并提供高度荧光稳定的羟基蒽醌。 尽管可以解释在用作细胞模型的烟草 BY-2 细胞中观察到的强烈可见荧光发射,但在本研究中进行的光化学反应期间从未观察到这些偶联产物。我们的数据表明,观察到的 ESIPT 诱导的荧光很可能对应于通过还原物质生成烷基化产物,如 β-巯基乙醇模型所证明的那样。总之, F-PDO作为一种新型(前)荧光探针,用于监测活细胞中的氧化还原过程和蛋白质烷基化。










































 京公网安备 11010802027423号
京公网安备 11010802027423号