当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Neuroprotective Agents, (+)-Lycibarbarine A and (−)-Lycibarbarine B
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00749 Arun K Ghosh 1 , Marc Harper 1 , William L Robinson 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00749 Arun K Ghosh 1 , Marc Harper 1 , William L Robinson 1
Affiliation
|
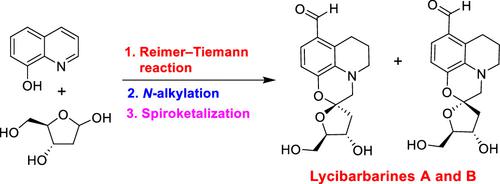
We describe the convergent total syntheses of lycibarbarines A and B which are potent neuroprotective agents recently isolated from the fruits of Lycium barbarum. The synthesis highlights the construction of a unique spiro oxazine heterocyclic motif imbedded in these natural products. The synthesis is accomplished from the commercially available 8-hydroxyquinaline and 2-deoxy-d-ribose as key starting materials. The synthesis features a Reimer–Tiemann reaction, selective amine alkylation with a keto tosylate derivative, and spiroketalization to form an oxazine core.
中文翻译:

神经保护剂 (+)-Lycibarbarine A 和 (−)-Lycibarbarine B 的全合成
我们描述了 lycibarbarines A 和 B 的聚合全合成,它们是最近从Lycium barbarum果实中分离出来的有效神经保护剂。该合成突出了嵌入这些天然产物中的独特螺恶嗪杂环基序的构建。该合成以市售的 8-羟基喹啉和 2-脱氧-d-核糖为关键起始原料完成。该合成过程包括 Reimer-Tiemann 反应、用酮基甲苯磺酸酯衍生物进行选择性胺烷基化,以及螺缩酮化形成恶嗪核。
更新日期:2023-06-02
中文翻译:

神经保护剂 (+)-Lycibarbarine A 和 (−)-Lycibarbarine B 的全合成
我们描述了 lycibarbarines A 和 B 的聚合全合成,它们是最近从Lycium barbarum果实中分离出来的有效神经保护剂。该合成突出了嵌入这些天然产物中的独特螺恶嗪杂环基序的构建。该合成以市售的 8-羟基喹啉和 2-脱氧-d-核糖为关键起始原料完成。该合成过程包括 Reimer-Tiemann 反应、用酮基甲苯磺酸酯衍生物进行选择性胺烷基化,以及螺缩酮化形成恶嗪核。











































 京公网安备 11010802027423号
京公网安备 11010802027423号