当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Oxidation of Methane to Methanol over Au/H-MOR
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-02 , DOI: 10.1021/jacs.3c04260 Wangyang Wang 1 , Wei Zhou 1, 2 , Yuchen Tang 1 , Weicheng Cao 2, 3 , Scott R Docherty 2 , Fangwei Wu 1 , Kang Cheng 1 , Qinghong Zhang 1 , Christophe Copéret 2 , Ye Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-02 , DOI: 10.1021/jacs.3c04260 Wangyang Wang 1 , Wei Zhou 1, 2 , Yuchen Tang 1 , Weicheng Cao 2, 3 , Scott R Docherty 2 , Fangwei Wu 1 , Kang Cheng 1 , Qinghong Zhang 1 , Christophe Copéret 2 , Ye Wang 1
Affiliation
|
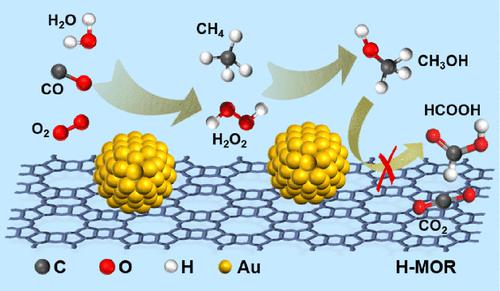
Selective oxidation of methane to methanol by dioxygen (O2) is an appealing route for upgrading abundant methane resource and represents one of the most challenging reactions in chemistry due to the overwhelmingly higher reactivity of the product (methanol) versus the reactant (methane). Here, we report that gold nanoparticles dispersed on mordenite efficiently catalyze the selective oxidation of methane to methanol by molecular oxygen in aqueous medium in the presence of carbon monoxide. The methanol productivity reaches 1300 μmol gcat–1 h–1 or 280 mmol gAu–1 h–1 with 75% selectivity at 150 °C, outperforming most catalysts reported under comparable conditions. Both hydroxyl radicals and hydroperoxide species participate in the activation and conversion of methane, while it is shown that the lower affinity of methanol on gold mainly accounts for higher methanol selectivity.
中文翻译:

Au/H-MOR 上甲烷选择性氧化成甲醇
通过双氧 (O 2 ) 将甲烷选择性氧化为甲醇是升级丰富的甲烷资源的一种有吸引力的途径,并且由于产物(甲醇)相对于反应物(甲烷)的反应性要高得多,因此代表了化学中最具挑战性的反应之一。在这里,我们报告分散在丝光沸石上的金纳米粒子在一氧化碳存在的情况下有效地催化水介质中分子氧将甲烷选择性氧化为甲醇。甲醇生产率达到 1300 μmol g cat –1 h –1或 280 mmol g Au –1 h –1在 150 °C 时具有 75% 的选择性,优于在可比条件下报道的大多数催化剂。羟基自由基和氢过氧化物物种均参与甲烷的活化和转化,同时表明甲醇对金的亲和力较低主要是甲醇选择性较高的原因。
更新日期:2023-06-02
中文翻译:

Au/H-MOR 上甲烷选择性氧化成甲醇
通过双氧 (O 2 ) 将甲烷选择性氧化为甲醇是升级丰富的甲烷资源的一种有吸引力的途径,并且由于产物(甲醇)相对于反应物(甲烷)的反应性要高得多,因此代表了化学中最具挑战性的反应之一。在这里,我们报告分散在丝光沸石上的金纳米粒子在一氧化碳存在的情况下有效地催化水介质中分子氧将甲烷选择性氧化为甲醇。甲醇生产率达到 1300 μmol g cat –1 h –1或 280 mmol g Au –1 h –1在 150 °C 时具有 75% 的选择性,优于在可比条件下报道的大多数催化剂。羟基自由基和氢过氧化物物种均参与甲烷的活化和转化,同时表明甲醇对金的亲和力较低主要是甲醇选择性较高的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号