当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of the Sub-oxide Sc4Au2O1–x and the Drastically Negative 27Al NMR Shift in Sc2Al
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.inorgchem.3c01097 Elias C J Gießelmann 1 , Mathis Radzieowski 2 , Samir F Matar 3 , Oliver Janka 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.inorgchem.3c01097 Elias C J Gießelmann 1 , Mathis Radzieowski 2 , Samir F Matar 3 , Oliver Janka 1
Affiliation
|
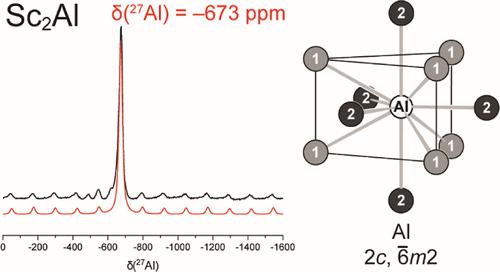
During attempts to synthesize Sc4AuAl in the cubic Gd4RhIn-type structure, the solid solution Sc2Au0.5Al0.5 in the PbCl2-type structure formed instead. Subsequently, the solid solution Sc2Au1–xAlx was investigated with respect to its existence range along with the structure types formed for different compositions with x = 0, 0.25, 0.5, 0.75, and 1. According to X-ray powder diffraction studies, Sc2Al and nominal Sc2Au0.25Al0.75 crystallized in the hexagonal Ni2In-type structure (P63/mmc), while Sc2Au0.5Al0.5, Sc2Au0.75Al0.25, and Sc2Au were found to crystallize in the orthorhombic PbCl2-type structure (Pnma). The crystal structures of Sc2Au and Sc2Au0.59(1)Al0.41(1) were refined from single-crystal data (Sc2Au: a = 648.0(1), b = 467.2(1), c = 835.2(2) pm, wR2 = 0.0382, 535 F2 values, 25 variables; Sc2Au0.59(1)Al0.41(1): a = 632.48(5), b = 472.16(3), c = 848.67(6) pm, wR2 = 0.0484, 540 F2 values, 21 variables). Contamination with air during the synthesis of Sc2Au led to the discovery of a compound adopting the cubic W4Co2C-type structure (stuffed cubic Ti2Ni type). Using Sc2O3 as a defined oxygen source led to samples with high amounts of Sc4Au2O1–x. All intermetallic compounds exhibited Pauli paramagnetic behavior in the investigated temperature range of 2.1 to 300 K, and no superconductivity was observed at low temperatures and low fields. Sc2Au and Sc2Al were investigated by 27Al and 45Sc solid-state NMR investigations. For Sc2Al, one signal was found in the 27Al NMR spectra in line with the crystal structure; however, an extremely negative resonance shift of δ = −673 ppm was observed. In both compounds, two Sc resonances were observed, in line with the proposed crystal structure. Finally, it was observed that the stability of Sc2Au in air is limited. This was investigated via thermal analysis and (temperature-dependent) powder X-ray diffraction. DFT calculations helped in assessing charge analysis, electronic properties, and chemical bonding.
中文翻译:

低氧化物 Sc4Au2O1–x 的形成以及 Sc2Al 中 27Al NMR 的大幅负位移
在尝试合成立方Gd 4 RhIn型结构中的Sc 4 AuAl期间,反而形成了PbCl 2型结构中的固溶体Sc 2 Au 0.5 Al 0.5 。随后,研究了固溶体Sc 2 Au 1– x Al x的存在范围以及x = 0、0.25、0.5、0.75和1的不同成分形成的结构类型。根据X射线粉末衍射研究,Sc 2 Al 和标称 Sc 2 Au 0.25 Al 0.75结晶在六方 Ni 中2 In型结构( P 6 3 / mmc ),而Sc 2 Au 0.5 Al 0.5、Sc 2 Au 0.75 Al 0.25和Sc 2 Au被发现以斜方PbCl 2型结构( Pnma )结晶。根据单晶数据精修了Sc 2 Au和Sc 2 Au 0.59(1) Al 0.41(1)的晶体结构(Sc 2 Au: a = 648.0(1), b = 467.2(1), c = 835.2( 2)下午,wR 2 = 0.0382,535 个F 2值,25 个变量;Sc 2 Au 0.59(1) Al 0.41(1):a = 632.48(5),b = 472.16(3),c = 848.67(6) pm,wR 2 = 0.0484,540 F 2值,21 个变量)。Sc 2 Au合成过程中的空气污染导致发现了采用立方W 4 Co 2 C型结构的化合物(填充立方Ti 2 Ni型)。使用 Sc 2 O 3作为确定的氧源可得到含有大量 Sc 4的样品Au 2 O 1– x。所有金属间化合物在2.1至300 K的研究温度范围内均表现出泡利顺磁行为,并且在低温和低场下没有观察到超导性。Sc 2 Au和Sc 2 Al通过27 Al和45 Sc固态NMR研究进行了研究。对于 Sc 2 Al,在27中发现了一个信号Al NMR谱与晶体结构相符;然而,观察到 δ = -673 ppm 的极端负共振位移。在这两种化合物中,观察到两个 Sc 共振,与所提出的晶体结构一致。最后,观察到Sc 2 Au在空气中的稳定性是有限的。这是通过热分析和(温度相关)粉末 X 射线衍射进行研究的。DFT 计算有助于评估电荷分析、电子特性和化学键合。
更新日期:2023-06-02
中文翻译:

低氧化物 Sc4Au2O1–x 的形成以及 Sc2Al 中 27Al NMR 的大幅负位移
在尝试合成立方Gd 4 RhIn型结构中的Sc 4 AuAl期间,反而形成了PbCl 2型结构中的固溶体Sc 2 Au 0.5 Al 0.5 。随后,研究了固溶体Sc 2 Au 1– x Al x的存在范围以及x = 0、0.25、0.5、0.75和1的不同成分形成的结构类型。根据X射线粉末衍射研究,Sc 2 Al 和标称 Sc 2 Au 0.25 Al 0.75结晶在六方 Ni 中2 In型结构( P 6 3 / mmc ),而Sc 2 Au 0.5 Al 0.5、Sc 2 Au 0.75 Al 0.25和Sc 2 Au被发现以斜方PbCl 2型结构( Pnma )结晶。根据单晶数据精修了Sc 2 Au和Sc 2 Au 0.59(1) Al 0.41(1)的晶体结构(Sc 2 Au: a = 648.0(1), b = 467.2(1), c = 835.2( 2)下午,wR 2 = 0.0382,535 个F 2值,25 个变量;Sc 2 Au 0.59(1) Al 0.41(1):a = 632.48(5),b = 472.16(3),c = 848.67(6) pm,wR 2 = 0.0484,540 F 2值,21 个变量)。Sc 2 Au合成过程中的空气污染导致发现了采用立方W 4 Co 2 C型结构的化合物(填充立方Ti 2 Ni型)。使用 Sc 2 O 3作为确定的氧源可得到含有大量 Sc 4的样品Au 2 O 1– x。所有金属间化合物在2.1至300 K的研究温度范围内均表现出泡利顺磁行为,并且在低温和低场下没有观察到超导性。Sc 2 Au和Sc 2 Al通过27 Al和45 Sc固态NMR研究进行了研究。对于 Sc 2 Al,在27中发现了一个信号Al NMR谱与晶体结构相符;然而,观察到 δ = -673 ppm 的极端负共振位移。在这两种化合物中,观察到两个 Sc 共振,与所提出的晶体结构一致。最后,观察到Sc 2 Au在空气中的稳定性是有限的。这是通过热分析和(温度相关)粉末 X 射线衍射进行研究的。DFT 计算有助于评估电荷分析、电子特性和化学键合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号