当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of 1,2-Azaborinine, a BN-Benzyne, with Organic π Systems
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00401 Divanshu Gupta 1 , Holger F Bettinger 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00401 Divanshu Gupta 1 , Holger F Bettinger 1
Affiliation
|
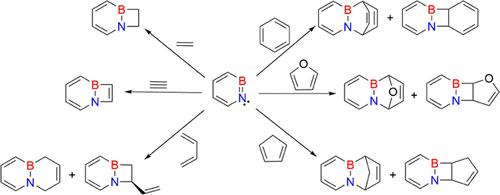
Ortho-benzyne and 1,2-azaborinine are related by the formal exchange of the CC triple bond by the isoelectronic BN unit. The (2 + 2) and (2 + 4) cycloaddition reactions of 1,2-azaborinine with the different organic π systems (ethene, ethyne, 1,3-butadiene, 1,3-cyclopentadiene, furan, benzene) were examined computationally using density functional, second-order perturbation, and coupled-cluster methods. All reactions of 1,2-azaborinine with the studied substrates are highly exothermic and involve the formation of Lewis acid–base complexes of 1,2-azaborinine and respective π systems. The interaction between the π bond of the substrates and the empty p orbital of the boron atom in these complexes is remarkably strong, resulting in two-step mechanisms for the (2 + 2) and (2 + 4) cycloaddition reactions. Cycloaddition reactions have lower barriers than CH insertion reactions, and (2 + 4) reactions are favored over (2 + 2) cycloadditions.
中文翻译:

1,2-氮杂硼啉(一种 BN-苯炔)与有机 π 系统的反应
邻苯炔和 1,2-氮杂硼啉通过等电子 BN 单元的 CC 三键形式交换而关联。通过计算检查了 1,2-氮杂硼啉与不同有机 π 体系(乙烯、乙炔、1,3-丁二烯、1,3-环戊二烯、呋喃、苯)的 (2 + 2) 和 (2 + 4) 环加成反应使用密度泛函、二阶扰动和耦合聚类方法。1,2-氮杂硼烷与所研究的底物的所有反应都是高度放热的,并且涉及1,2-氮杂硼烷和相应π体系的路易斯酸碱配合物的形成。这些配合物中底物的 π 键与硼原子的空 p 轨道之间的相互作用非常强,导致 (2 + 2) 和 (2 + 4) 环加成反应的两步机制。
更新日期:2023-06-02
中文翻译:

1,2-氮杂硼啉(一种 BN-苯炔)与有机 π 系统的反应
邻苯炔和 1,2-氮杂硼啉通过等电子 BN 单元的 CC 三键形式交换而关联。通过计算检查了 1,2-氮杂硼啉与不同有机 π 体系(乙烯、乙炔、1,3-丁二烯、1,3-环戊二烯、呋喃、苯)的 (2 + 2) 和 (2 + 4) 环加成反应使用密度泛函、二阶扰动和耦合聚类方法。1,2-氮杂硼烷与所研究的底物的所有反应都是高度放热的,并且涉及1,2-氮杂硼烷和相应π体系的路易斯酸碱配合物的形成。这些配合物中底物的 π 键与硼原子的空 p 轨道之间的相互作用非常强,导致 (2 + 2) 和 (2 + 4) 环加成反应的两步机制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号