当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Collaborative Mechanistic Effects between Vanadia and Titania during the Oxidative Dehydrogenation of Propane Investigated by Operando and Transient Spectroscopies
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acscatal.3c01404 Leon Schumacher 1 , Johannes Pfeiffer 1 , Jun Shen 1 , Torsten Gutmann 1 , Hergen Breitzke 1 , Gerd Buntkowsky 1 , Kathrin Hofmann 1 , Christian Hess 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-06-02 , DOI: 10.1021/acscatal.3c01404 Leon Schumacher 1 , Johannes Pfeiffer 1 , Jun Shen 1 , Torsten Gutmann 1 , Hergen Breitzke 1 , Gerd Buntkowsky 1 , Kathrin Hofmann 1 , Christian Hess 1
Affiliation
|
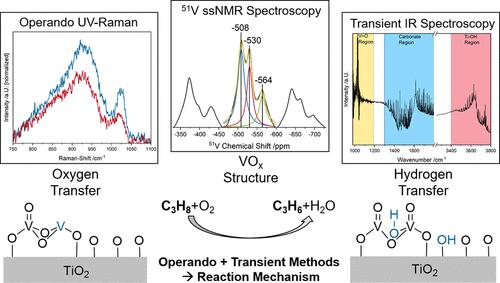
The oxidative dehydrogenation (ODH) of propane is of great technical importance, and supported VOx catalysts have shown promising properties for the reaction. One of the most prominent and active supports is titania, which exhibits a high activity but many questions regarding the catalyst system are still in debate. In this study, we elucidate the mechanism of the propane ODH reaction over VOx/TiO2, using P25 and ALD (atomic layer deposition) synthesized TiO2/SBA-15 as a support, with X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), 51V solid-state (ss)NMR, operando multiwavelength Raman, operando UV–vis, and transient IR spectroscopies. Bare titania shows a small conversion, leading to carbon formation, and the reaction occurs at the interface between anatase and rutile. In comparison, in VOx/TiO2 catalysts, the activity shifts from titania to vanadia sites. UV-Raman spectroscopy and structural characterization data revealed the reaction to involve preferentially the V═O bonds of dimeric species rather than doubly bridged V–O–V bonds, which leads to higher propene selectivities. The active vanadium site shows a nuclearity-dependent behavior; that is, at higher loadings, when oligomeric vanadia is present, it shifts from V═O bonds to linear V–O–V bonds in oligomers, leading to less selective oxidation due to the better reducibility. Our operando/transient spectroscopic results demonstrate the direct participation of the titania support in the reaction by influencing the degree of vanadia oligomerization and enabling rapid hydrogen transfer from propane to vanadia via Ti–OH groups on anatase, accelerating the rate-determining step of the initial C–H bond breakage. The broader applicability of the results is confirmed by the behavior of the ALD-synthesized sample, which resembles that of P25. Our results highlight the detailed level of mechanistic understanding accessible from multiple spectroscopic approaches, which can be readily transferred to other materials and/or reactions.
中文翻译:

Operando 和瞬态光谱研究丙烷氧化脱氢过程中 Vanadia 和 Titania 的协同机制效应
丙烷的氧化脱氢 (ODH) 具有重要的技术意义,负载型 VOx催化剂已显示出有前途的反应性能。最突出和最活跃的载体之一是二氧化钛,它表现出高活性,但有关催化剂系统的许多问题仍在争论中。在本研究中,我们以 P25 和 ALD(原子层沉积)合成的 TiO 2 /SBA-15 为载体,利用 X 射线光电子能谱(XPS)阐明了 VO x /TiO 2上丙烷 ODH 反应的机理, X 射线衍射 (XRD),51V 固态 (ss) NMR、原位多波长拉曼、原位 UV-vis 和瞬态 IR 光谱。裸露的二氧化钛显示出小的转化,导致碳形成,并且反应发生在锐钛矿和金红石之间的界面处。相比之下,在 VO x /TiO 2催化剂,活性从二氧化钛转移到氧化钒位点。紫外-拉曼光谱和结构表征数据表明,反应优先涉及二聚体的 V=O 键,而不是双桥接的 V-O-V 键,这导致更高的丙烯选择性。活性钒位点显示出核度依赖行为;也就是说,在更高的负载量下,当存在低聚钒时,它从 V=O 键转变为低聚物中的线性 V-O-V 键,由于更好的还原性,导致选择性氧化更少。我们的原位/瞬态光谱结果表明,二氧化钛载体通过影响钒齐聚的程度并通过锐钛矿上的 Ti-OH 基团使氢快速从丙烷转移到钒,从而直接参与反应,加速初始 C-H 键断裂的决速步骤。ALD 合成样品的行为证实了结果的更广泛适用性,该样品类似于 P25。我们的结果突出了可从多种光谱方法获得的详细机理理解水平,这些方法可以很容易地转移到其他材料和/或反应中。
更新日期:2023-06-02
中文翻译:

Operando 和瞬态光谱研究丙烷氧化脱氢过程中 Vanadia 和 Titania 的协同机制效应
丙烷的氧化脱氢 (ODH) 具有重要的技术意义,负载型 VOx催化剂已显示出有前途的反应性能。最突出和最活跃的载体之一是二氧化钛,它表现出高活性,但有关催化剂系统的许多问题仍在争论中。在本研究中,我们以 P25 和 ALD(原子层沉积)合成的 TiO 2 /SBA-15 为载体,利用 X 射线光电子能谱(XPS)阐明了 VO x /TiO 2上丙烷 ODH 反应的机理, X 射线衍射 (XRD),51V 固态 (ss) NMR、原位多波长拉曼、原位 UV-vis 和瞬态 IR 光谱。裸露的二氧化钛显示出小的转化,导致碳形成,并且反应发生在锐钛矿和金红石之间的界面处。相比之下,在 VO x /TiO 2催化剂,活性从二氧化钛转移到氧化钒位点。紫外-拉曼光谱和结构表征数据表明,反应优先涉及二聚体的 V=O 键,而不是双桥接的 V-O-V 键,这导致更高的丙烯选择性。活性钒位点显示出核度依赖行为;也就是说,在更高的负载量下,当存在低聚钒时,它从 V=O 键转变为低聚物中的线性 V-O-V 键,由于更好的还原性,导致选择性氧化更少。我们的原位/瞬态光谱结果表明,二氧化钛载体通过影响钒齐聚的程度并通过锐钛矿上的 Ti-OH 基团使氢快速从丙烷转移到钒,从而直接参与反应,加速初始 C-H 键断裂的决速步骤。ALD 合成样品的行为证实了结果的更广泛适用性,该样品类似于 P25。我们的结果突出了可从多种光谱方法获得的详细机理理解水平,这些方法可以很容易地转移到其他材料和/或反应中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号