Structure ( IF 5.7 ) Pub Date : 2023-06-01 , DOI: 10.1016/j.str.2023.05.006 Remy A Yovanno 1 , Alvin Yu 2 , Tyler J Wied 1 , Albert Y Lau 1
|
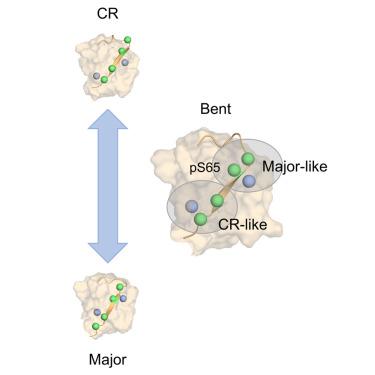
Ubiquitin phosphorylation at Ser65 increases the population of a rare C-terminally retracted (CR) conformation. Transition between the Major and CR ubiquitin conformations is critical for promoting mitochondrial degradation. The mechanisms by which the Major and CR conformations of Ser65-phosphorylated (pSer65) ubiquitin interconvert, however, remain unresolved. Here, we perform all-atom molecular dynamics simulations using the string method with swarms of trajectories to calculate the lowest free-energy path between these two conformers. Our analysis reveals the existence of a Bent intermediate in which the C-terminal residues of the β5 strand shift to resemble the CR conformation, while pSer65 retains contacts resembling the Major conformation. This stable intermediate was reproduced in well-tempered metadynamics calculations but was less stable for a Gln2Ala mutant that disrupts contacts with pSer65. Lastly, dynamical network modeling reveals that the transition from the Major to CR conformations involves a decoupling of residues near pSer65 from the adjacent β1 strand.
中文翻译:

Ser65 磷酸化调节泛素构象动力学
Ser65 处的泛素磷酸化会增加罕见的 C 末端回缩 (CR) 构象的数量。主要泛素和 CR 泛素构象之间的转变对于促进线粒体降解至关重要。然而,Ser65 磷酸化 (pSer65) 泛素的主要构象和 CR 构象相互转换的机制仍未解决。在这里,我们使用弦法和轨迹群进行全原子分子动力学模拟,以计算这两个构象异构体之间的最低自由能路径。我们的分析揭示了 Bent 中间体的存在,其中 β5 链的 C 端残基转变为类似于 CR 构象,而 pSer65 保留了类似于 Major 构象的接触。这种稳定的中间体在经过磨炼的情况下得到了再现元动力学计算,但对于破坏与 pSer65 接触的 Gln2Ala 突变体来说不太稳定。最后,动态网络模型表明,从主要构象到 CR 构象的转变涉及 pSer65 附近的残基与相邻 β1 链的解偶联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号