当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reaction between Alkylidene Pyrazolones and Allyl Ketones: Access to Tetrahydropyrano[2,3-c]pyrazoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.joc.3c01063 Nimisha Bania 1 , Dipankar Barman 1 , Subhas Chandra Pan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.joc.3c01063 Nimisha Bania 1 , Dipankar Barman 1 , Subhas Chandra Pan 1
Affiliation
|
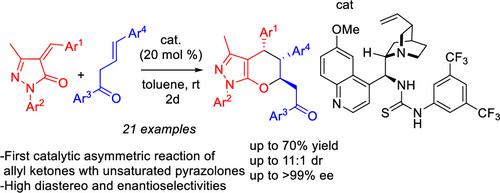
Herein we report a catalytic asymmetric inverse-electron-demand Diels–Alder reaction between alkylidene pyrazolones and allyl ketones. Allyl ketone gets activated by a bifunctional thiourea catalyst and acts as a dienolate in this reaction. The trisubstituted tetrahydropyrano[2,3-c]pyrazoles were obtained in moderate to good yields with high diastereo- and enantioselectivities. Few applications, including a decarbonylation reaction, have been demonstrated.
中文翻译:

亚烷基吡唑啉酮和烯丙基酮之间的有机催化不对称逆电子需求狄尔斯-阿尔德反应:获得四氢吡喃并[2,3-c]吡唑
在此,我们报道了亚烷基吡唑啉酮和烯丙基酮之间的催化不对称逆电子需求狄尔斯-阿尔德反应。烯丙基酮被双功能硫脲催化剂活化,并在此反应中充当二烯醇化物。三取代的四氢吡喃并[2,3- c ]吡唑以中等至良好的产率获得,具有高非对映选择性和对映选择性。已经证实的应用很少,包括脱羰反应。
更新日期:2023-06-01
中文翻译:

亚烷基吡唑啉酮和烯丙基酮之间的有机催化不对称逆电子需求狄尔斯-阿尔德反应:获得四氢吡喃并[2,3-c]吡唑
在此,我们报道了亚烷基吡唑啉酮和烯丙基酮之间的催化不对称逆电子需求狄尔斯-阿尔德反应。烯丙基酮被双功能硫脲催化剂活化,并在此反应中充当二烯醇化物。三取代的四氢吡喃并[2,3- c ]吡唑以中等至良好的产率获得,具有高非对映选择性和对映选择性。已经证实的应用很少,包括脱羰反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号