当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability Islands and the Folding Cooperativity of a Seven-Repeat Array from Topoisomerase V
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-01 , DOI: 10.1021/jacs.3c02193 Mark Petersen 1 , Rebecca Fang 1 , Ananya Majumdar 2 , Doug Barrick 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-01 , DOI: 10.1021/jacs.3c02193 Mark Petersen 1 , Rebecca Fang 1 , Ananya Majumdar 2 , Doug Barrick 1
Affiliation
|
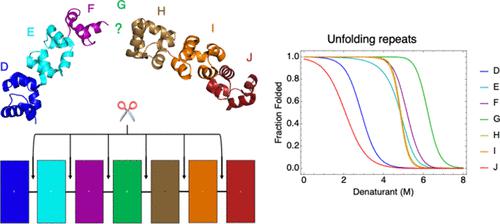
Cooperativity is a central feature of protein folding, but the thermodynamic and structural origins of cooperativity remain poorly understood. To quantify cooperativity, we measured guanidine-induced unfolding transitions of single helix–hairpin–helix (HhH)2 repeats and tandem pairs from a seven-repeat segment of Methanopyrus kandleri Topoisomerase V (Topo V) to determine intrinsic repeat stability and interfacial free energies between repeats. Most single-repeat constructs are folded and stable; moreover, several pairs have unfolding midpoints that exceed midpoints of the single repeats they comprise, demonstrating favorable coupling between repeats. Analyzing unfolding transitions with a modified Ising model, we find a broad range of intrinsic and interfacial free energies. Surprisingly, the G repeat, which lacks density in the crystal structure of Topo V without DNA, is the most stable repeat in the array. Using nuclear magnetic resonance spectroscopy, we demonstrate that the isolated G repeat adopts a canonical (HhH)2 fold and forms an ordered interface with the F-repeat but not with the H repeat. Using parameters from our paired Ising fit, we built a partition function for the seven-repeat array. The multistate unfolding transition predicted from this partition function is in excellent agreement with the experimental unfolding transition, providing strong justification for the nearest-neighbor model. The seven-repeat partition function predicts a native state in which three independent segments (“stability islands”) of interacting repeats are separated by two unstable interfaces. We confirm this segmented architecture by measuring the unfolding transition of an equimolar mixture of these three separate polypeptides. This segmented structural organization may facilitate wrapping around DNA.
中文翻译:

拓扑异构酶 V 七次重复阵列的稳定性岛和折叠协同性
协同性是蛋白质折叠的核心特征,但协同性的热力学和结构起源仍然知之甚少。为了量化协同性,我们测量了来自Methanopyrus kandleri拓扑异构酶 V (Topo V) 七重复片段的单螺旋 - 发夹 - 螺旋 (HhH) 2重复和串联对的胍诱导的展开转变,以确定内在重复稳定性和界面自由能重复之间。大多数单重复构建体都是折叠且稳定的;此外,有几对的展开中点超过了它们所组成的单个重复的中点,这表明重复之间存在良好的耦合。使用修正的伊辛模型分析展开转变,我们发现了广泛的内在和界面自由能。令人惊讶的是,在不含 DNA 的 Topo V 晶体结构中缺乏密度的 G 重复序列是阵列中最稳定的重复序列。使用核磁共振波谱,我们证明孤立的 G 重复采用规范 (HhH) 2折叠,并与 F 重复但不与 H 重复形成有序界面。使用配对伊辛拟合中的参数,我们为七次重复阵列构建了一个配分函数。从该配分函数预测的多态展开转变与实验展开转变非常一致,为最近邻模型提供了强有力的证明。七重复分区函数预测了一种天然状态,其中相互作用重复的三个独立片段(“稳定岛”)被两个不稳定界面分开。我们通过测量这三种独立多肽的等摩尔混合物的展开转变来确认这种分段结构。 这种分段的结构组织可能有助于包裹 DNA。
更新日期:2023-06-01
中文翻译:

拓扑异构酶 V 七次重复阵列的稳定性岛和折叠协同性
协同性是蛋白质折叠的核心特征,但协同性的热力学和结构起源仍然知之甚少。为了量化协同性,我们测量了来自Methanopyrus kandleri拓扑异构酶 V (Topo V) 七重复片段的单螺旋 - 发夹 - 螺旋 (HhH) 2重复和串联对的胍诱导的展开转变,以确定内在重复稳定性和界面自由能重复之间。大多数单重复构建体都是折叠且稳定的;此外,有几对的展开中点超过了它们所组成的单个重复的中点,这表明重复之间存在良好的耦合。使用修正的伊辛模型分析展开转变,我们发现了广泛的内在和界面自由能。令人惊讶的是,在不含 DNA 的 Topo V 晶体结构中缺乏密度的 G 重复序列是阵列中最稳定的重复序列。使用核磁共振波谱,我们证明孤立的 G 重复采用规范 (HhH) 2折叠,并与 F 重复但不与 H 重复形成有序界面。使用配对伊辛拟合中的参数,我们为七次重复阵列构建了一个配分函数。从该配分函数预测的多态展开转变与实验展开转变非常一致,为最近邻模型提供了强有力的证明。七重复分区函数预测了一种天然状态,其中相互作用重复的三个独立片段(“稳定岛”)被两个不稳定界面分开。我们通过测量这三种独立多肽的等摩尔混合物的展开转变来确认这种分段结构。 这种分段的结构组织可能有助于包裹 DNA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号